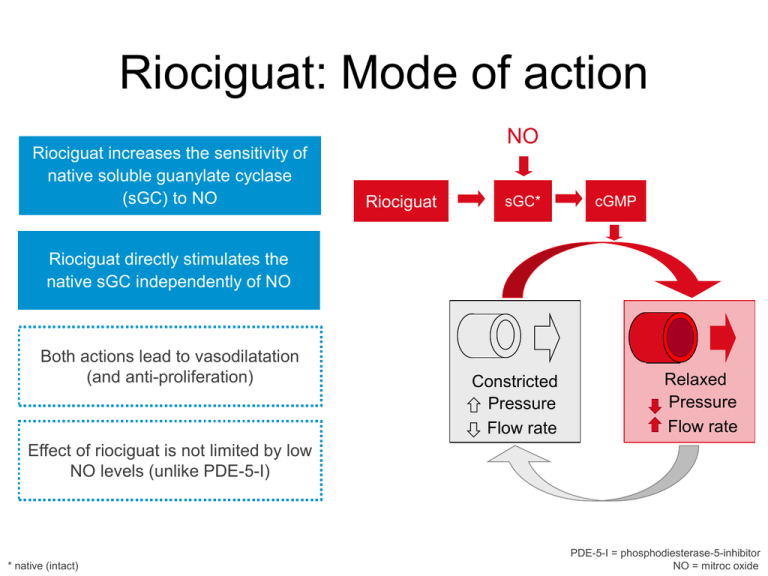
Riociguat: Mode of action
Riociguat increases the sensitivity of
native soluble guanylate cyclase
(sGC) to NO
NO
Riociguat
sGC*
cGMP
Riociguat directly stimulates the
native sGC independently of NO
Both actions lead to vasodilatation
(and anti-proliferation)
Constricted
Pressure
Flow rate
Relaxed
Pressure
Flow rate
Effect of riociguat is not limited by low
NO levels (unlike PDE-5-I)
* native (intact)
PDE-5-I = phosphodiesterase-5-inhibitor
NO = mitroc oxide
Anti-remodeling effects of
riociguat
in a rat model of PH
Rats
20–70 µm percentage of
total vessel count
Vessel muscularization
100
*
*
†
80
60
40
20
0
MCT
Riociguat
*
*
N P
–
–
M
†
†
N P
M
*
N P
M
+
–
N P
M
+
–
MCT
21 days
+
+
MCT
35 days
*p < 0.05 versus control animals without PH; †p < 0.05 versus untreated animals with PH at day 35.
N, non-muscularized; P, partially muscularized; M, fully muscularized.
Schermuly et al., ERJ 2008
2
Haemodynamic effects of BAY 632521:
decrease in vascular resistance
BAY 63-2521
Pulmonary vascular resistance
1 mg study group
(n = 5)
Systemic vascular resistance
2.5 mg study group
(n = 10)
-5
-10
-15
-20
-25
-30
-35
-45
*p < 0.05
0
Point estimate of decrease from baseline (%)
Point estimate of decrease from baseline (%)
0
-40
iNO
1 mg study group 2.5 mg study group
(n = 5)
(n = 10)
‡
§
*
‡
-5
‡p
-10
§p
< 0.001
< 0.0001
-15
-20
BAY 63-2521
-25
iNO
-30
-35
-40
§
-45
-50
§
§
§
Grimminger et al., ERJ 2009
3
Haemodynamic effects of
BAY 63-2521:
increase in cardiac index
Point estimate of increase from baseline (%)
45
§
§
50
§
iNO
§
§p
40
35
30
BAY 63-2521
25
iNO
20
15
10
5
0
1 mg study group 2.5 mg study group
(n = 5)
(n = 10)
Cardiac index
Grimminger et al., ERJ 2009
BAY 63-2521
< 0.0001
Riociguat phase 2 study
• Multicenter, open-label, individual dosetitration study
• Primary objective: to investigate the safety,
tolerability and feasibility of individual
titration of riociguat according to peripheral
systolic blood pressure
• Secondary objectives: to assess the
pharmacodynamics and pharmacokinetics
of riociguat
5
Dose titration scheme
• If trough SBP > 100 mmHg, increase dose (+0.5 mg t.i.d.)
• If trough SBP 90–100 mmHg, maintain dose
• If trough SBP < 90 mmHg without symptoms of hypotension,
reduce dose (–0.5 mg t.i.d.)
• If trough SBP < 90 mmHg with symptoms of hypotension,
restart after 24 hours with reduced dose (–0.5 mg t.i.d.)
2.5 mg t.i.d.
2.0 mg t.i.d.
1.5 mg t.i.d.
1 mg t.i.d.
Day 1
Week 2
Week 4
Week 6
6
Week 8
Week 12
Baseline demographics
Demographic variable
Total patients
PAH
CTEPH
Age (years)
Race
White
Sex
Men
Women
Body mass index (kg/m2)
n (%) or mean
75 (100%)
33 (44%)
42 (56%)
60.3 (range: 19–76)
75 (100%)
34 (45%)
41 (55%)
26.1 (SD: 4.4)
7
Baseline hemodynamic and
functional parameters
Parameter
mPAP (mmHg)
n (%) or mean ± SD
45.3 ± 10.8
CO (L/min)
RAP (mmHg)
PCWP (mmHg)
4.1 ± 1.1
6.6 ± 4.3
8.0 ± 4.2
PVR/SVR
NYHA class:
I
II
III
IV
6-minute walking
distance (m)
45.7 ± 15.7
0 (0%)
15 (21%)
56 (78%)
1 (1%)
354.4 ± 111.0
8
Six-minute walking distance:
all patients
CTEPH
n = 41
PAH
n = 31
All
n = 72
Change in 6-minute
walking distance (m)
100
80
60
40
20
0
Baseline
2
4
6
8
10
Duration of treatment (weeks)
Titration phase
Baseline values
PAH: 316.7 127.4; CTEPH: 382.9 88.1; All: 354.4 111.0
9
12
n = 20
n = 30
n = 50
PAH
CTEPH
All
0
–2
–4
–6
***
–8
***
–10
–12
–14
*
*p < 0.05; ***p < 0.001
Mean decrease from baseline in
pulmonary vascular resistance (dyn.s/cm5)
Mean decrease from baseline in
pulmonary arterial pressure (mmHg)
Pulmonary arterial pressure and
pulmonary vascular resistance
10
n = 19
n = 29
n = 48
PAH
CTEPH
All
0
–50
–100
–150
–200
–250
***
–300
–350
***
–400
–450
–500
***
Functional class
100%
Proportion of patients (%)
90%
80%
70%
60%
50%
40%
30%
20%
10%
0%
Baseline
12 weeks
PAH
n = 72
NYHA class IV
Baseline
12 weeks
CTEPH
n = 41
NYHA class III NYHA class II
Baseline
Total
n = 31
NYHA class I
CTEPH, chronic thromboembolic pulmonary hypertension; NYHA, New York Heart Association;
PAH, pulmonary arterial hypertension
11
12 weeks
Riociguat phase III clinical
program: PATENT -1 and -2
PATENT: Pulmonary Arterial Hypertension sGC-Stimulator Trial
Secondary Outcome Measures
Primary Outcome Measure
• Change from baseline in 6 Minute Walk
• Change from baseline in Pulmonary
Test after 16 weeks*
*Secondary outcome in extension, ** primary
outcome in extension;
p.o.: per os - oral; TID: three times daily; NT-pro
BNP: N-terminal pro brain natriuretic peptide; EQ-5D:
quality-of-life measures; MLHF-Q: Minnesota Living
with Heart Failure Questionnaire
•
Vascular Resistance (PVR), change from
baseline in WHO functional class, change
from baseline in NT-pro BNP, change from
baseline in Borg dyspnea, change from
baseline in EQ-5D and MLHF-Q, time to
clinical worsening
Safety**
Riociguat phase III clinical
program: CHEST -1 and -2
CHEST: Chronic Thromboembolic Pulmonary Hypertension sGC-Stimulator Trial
Primary Outcome Measure
•
Change from baseline in 6 Minute Walk
Test after 16 weeks*
*Secondary outcome in extension, ** primary outcome
in extension;
p.o.: per os - oral; TID: three times daily; NT-pro
BNP: N-terminal pro brain natriuretic peptide; EQ-5D:
quality-of-life measures; MLHF-Q: Minnesota Living
with Heart Failure Questionnaire
Secondary Outcome Measures
• Change from baseline in Pulmonary
•
Vascular Resistance (PVR), change from
baseline in WHO functional class, change
from baseline in NT-pro BNP, change from
baseline in Borg dyspnea, change from
baseline in EQ-5D and MLHF-Q, time to
clinical worsening
Safety**












