Honors Chapter 3 Measurement
advertisement

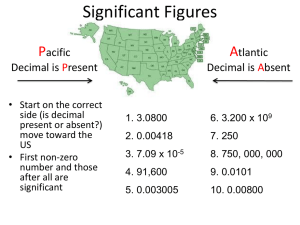
Chemistry Chapter 3 Scientific Measurement Qualitative Measurement •Gives results in a descriptive form •Nonnumeric Quantitative Measurement ►Gives form results in a definite ►Usually units as numbers and Scientific Notation • Shorthand way to express very large and very small numbers Example 4 10 3.6 x = 3.6 x 10 x 10 x 10 x 10 = 36 000 0.0081 = -3 8.1 x 10 Direction of decimal movement To the left is + To the right is - –Operations with numbers in scientific notation Multiplication –Multiply the numbers and then add the exponents Division –Divide the numbers and subtract the exponents Addition and subtraction Exponent must be the same to proceed Must move the decimal appropriately and then adjust the exponent Then you can solve the problem Measured values only as reliable as the instrument used to take the measurement! Uncertainty in measurement Accuracy Measure of how close a measurement comes to the actual or true value Precision –Measure of how close a series of measurements are to one another Pg. 64 Dartboard example In class: Pg. 97 #80 Evaluating the accuracy of a measurement • Percent error • Percent error = [error] X 100 accepted value Error - the difference between the accepted value and the experimental value (absolute value) • Experimental value – measured in the lab • Accepted value – correct value based on reliable references • Pg. 65 example Everyone understand so far? Good!!! Significant figures in measurements (sig figs) Rules page 66-67 Sample problems Pg. 68 Sig Figs in Calculations Rules for rounding Pg. 68 Page 69 Sample Solving problems with sig figs Multiplying and dividing with sig figs The answer you get must be rounded to the same number of sig figs as the measurement with the lowest number of sig figs (that you multiplied or divided) Example Multiply 4.610 feet by 1.7 feet. Express your answer in correct sig figs 4.610 x 1.7 = 7.837 How do you round it? 4.610 has 4 sig figs 1.7 has 2 sig figs Round answer to 2 sig figs Answer = 7.8 square feet Adding and Subtracting with sig figs When adding or subtracting measurements, the answer cannot have more certainly than the least certain measurement. Answer must have the same number of sig figs to the right of the decimal point as the measurement with the fewest sig figs to the right of the decimal point Example 4.271 grams (3 sig figs to the right of decimal) 2 grams (0 sig figs to the right of decimal) + 10.0 grams (1 sig fig to the right of decimal) 16.271 grams round 16 grams Handout practice – work with a partner! Grab a calculator SI System of Units •Page 73 Units of measurement •Table 3.1 • Metric system established in France in 1790 • SI Adopted by international agreement in 1960 Prefixes Page 74 Table 3.2 Length SI unit - meter (m) Pg. 74 Table 3.3 Volume Space occupied by any sample of matter L x W x H “Derived” unit Pg. 75 Table 3.4 • Volume of a cube 1m on each side • SI unit = m3 • More common to use Liter (L) = dm3 1 Liter the volume occupied by a cube 10 cm on each side 10 cm x 10 cm x 10 3 cm = 1000 cm 1000 3 cm =1L 1 dm = 10 cm 1 L = 1 3 dm 1 mL = 0.001 L 1000 mL = 1 L 1000 3 cm = 1000 mL = 1 L •Volumes for solids, liquids, gases change with change in temperature Much more dramatic with gases Measuring devices calibrated at 20oC Room temperature Mass • Difference between mass and weight • SI unit = Kilogram (kg) • 1 g = 0.001 kg • 1000 g = 1 kg • Pg. 76 Table 3.5 • Will show on board something special about H2O Temperature Scales Celsius Kelvin Absolute zero Kelvin scale explanation Heat measurement calorie Joule 1 cal = 4.184 J 1J = 0.2390 cal Unit Conversions Also called “factor labeling” How many inches in 2 feet? How many feet in 36 inches? You just did a unit conversion!!!!!! Look at board Must use correct “conversion factor” • 230 cm = ? m • Must know that 100 cm = 1m • Write possible conversion factors • 1m or 100 cm 100 cm 1m Write the number you are converting first Multiply it by the conversion factor that has the unit you want your answer to be in on the TOP This guarantees that you will divide or multiply when you are supposed to. • 230 cm x 1m 100 cm = 2.3 m The top and bottom units cancel out and the only unit left is the one you want you answer to be in!!!!! Groups!! Pg. 84-85 # 32-35 Two step conversions 4500 cm = ? km Derived units What does “derived” mean? A derived unit is a measurement unit created by multiplying or dividing other units Miles per hour words per minute Area Area Length x width ft x ft = ft2 ft2 is a derived unit (derived from two length units) m x m = m2 m2 is a derived unit (derived from two length units) Volume Length x width x height ft x ft x ft = ft3 m x m x m = m3 cm x cm x cm = cm3 Density Describes how dense something is How heavy it is for its size Density = mass divided by volume D=M V M=DxV V=M D Since you are dividing two different measurements, the unit for density is a DERIVED UNIT. Derived from a mass measurement and a volume measurement g/mL g/L Density problem Calculate the density of a substance with a mass of 24.3 g and a volume of 32.9 mL. Use the correct unit and the correct number of sig figs in your answer. D=M V D = 24.3 g 32.9 mL Ans. = 0.739 g/mL Problem What is the volume of an object with a density of 1.25 g/mL and a mass of 281 g? V=M D V = 281 g 1.25 g/mL g cancels, so units are mL for answer V = 225 mL Volume of irregularly shaped object Water displacement Go over hw