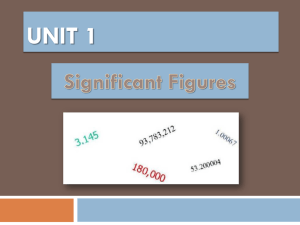
Measurement
advertisement

Chapter 2. Measurement Chemistry is a physical science, one that depends heavily on measurement to obtain quantitative data. Measurement is the determination of dimensions, capacity, quantity, or extent of something. Precision, Accuracy, Error Precision, Accuracy, Error Precision refers to the reproducibility of a given measurement. Accuracy refers to the agreement between a measurement (or average several measurements) to the true or accepted value. Errors are deviations (differences) between the true value and a measurement. They can be random or systematic. Uncertainty in Measurement All measurements contain some error, or uncertainty. Heisenberg's Uncertainty Principle is a natural law that states that the act of observing something changes it. In its mathematical form, it states the limits of accuracy in a measurement. Uncertainty in Measurement Generally, uncertainty is minimized (accuracy is maximized) by using equipment that is better designed, more expensive, and more demanding to use. Beaker 1 milliliter $3.00 Cylinder 0.1 milliliter $6.00 Buret 0.01 milliliter $90.00 Uncertainty in Measurement Uncertainty in Measurement Numbers express uncertainty. Exact numbers contain no uncertainty. They are obtained by counting objects (integers) or are defined, as in some conversion factors. Inexact numbers contain uncertainty. They are obtained from measurements. The uncertainty in a measurement is shown by the number of digits recorded. Uncertainty in Measurement Significant figures are the digits in a measurement that are known with certainty, plus one digit that is uncertain. Last digit is uncertain. 14.3 gallons 0.1 gal (13 oz) 14.325 gallons 0.001 gal (¾ tsp) Uncertainty in Measurement Significant figures allow us to estimate the uncertainty of a value: 10 +/– 1 1 x 100% = 10% 10 10.0 +/– 0.1 0.1 x 100% = 1% 10.0 10.00 +/– 0.01 0.01 x 100% = 0.1% 10.00 Uncertainty in Measurement Significant figures and measurements: With digital instruments (reading is numbers) record all digits from the instrument. The last digit is uncertain and may fluctuate. With analog instruments (reading is a scale) "read between the lines" for last digit. That digit is an estimate, and contains uncertainty. Working with "Sig Figs" How many significant figures are there in each of these numbers? 14.3 gallons 14.325 gallons 14.0 gallons 0.025 gallons Working with "Sig Figs" In a number from a measurement: All nonzero digits are significant. Internal zeroes are significant. Leading zeroes are not significant. Trailing zeroes are significant if there is a decimal point in the number. Working with "Sig Figs" How many significant figures in each number? 1800 1800. 1800.00 Working with "Sig Figs" Why does this matter? Because we usually do calculations on measurements. I ride my bike to school. The distance is 4.1 miles. The other day it took 25.7 minutes. What was my average speed, in miles per minute, and in miles per hour? 4.1 mi = 0.1595440793 mi 25.7 min min 4.1 mi = 0.16 mi = 9.6 mi 25.7 min min hr Working with "Sig Figs" Why did I convert 0.1595440793 mi/min to 0.16 mi/min and not something else? Rules for working with significant figures: Rounding Sig figs in calculations Working with "Sig Figs" Rounding numbers is disposing of nonsignificant digits Only do this in calculations! Do not round off measurements! Working with "Sig Figs" Rules for rounding: 1. Decide how many digits are significant. 2. Underline them (till you catch on) 3. Look at digit to right of last underlined digit a. If it's 1 4, drop it. b. If it's 5 9, add 1 to last sig. fig. Working with "Sig Figs" Examples: Round the following values to 3 sig. figs. 27.428 39.572 0.01565 Calculating with "Sig Figs" Rules for Calculations: 1. Multiplication and division: The result has the same number of sig figs as the value with the fewest sig figs. 4.1 mi = 0.1595440793 mi 25.7 min min 4.1 mi = 0.16 mi = 9.6 mi 25.7 min min hr Calculating with "Sig Figs" Rules for Calculations: 2. Addition and Subtraction: The result has the same uncertainty as the value with the greatest uncertainty. Keep digits as far to the right as all values have sig figs. 23.456 + 82.9 106.356 ~ 106.4 100.423 -100.312 0.111 Calculating with "Sig Figs" Examples: Calculate the quantities, and round to the correct number of significant figures: 15.86 x 2.34 12.356 = 907.369 – 15.5 = (143.289 – 139.143) = 187.467 Calculating with "Sig Figs" Rules for Calculations: 4. Working with exact and inexact numbers: Exact numbers don't have sig figs because they don’t introduce uncertainty. Just use sig figs in inexact numbers 0.16 mi x 60 min = 9.6 mi min hr hr 60 min/hour is an exact conversion Calculating with "Sig Figs" Rules for Calculations: 5. If your calculator gives you fewer sig figs than the value should have, add zeroes 0.465 x 0.200 = 0.0930 4.389 – 2.589 = 1.800 Calculating with "Sig Figs" Rules for Calculations: 6. If your calculator gives you more digits to the left of the decimal than are significant, use scientific notation. 75.3 x 24.8 x 675 = 1260522 = 1.26 x 106 Scientific Notation Scientific notation is a numerical system in which a decimal number is expressed as the product of a number between 1 and 10, and 10 raised to a power. 1260522 = 1.260522 x 1,000,000 = 1.260522 x 106 = 1.26 x 106 1.26 is the coefficient 106 is the exponential term Scientific Notation Scientific notation is useful for expressing very large or very small numbers. 93,000,000 miles from earth to sun 9.3 x 107 miles 0.000 000 000 000 000 000 000 030 grams 3.0 x 10-23 grams is mass of 1 water molecule Scientific Notation When should scientific notation be used? 1. It MUST be used if you have more digits than significant digits to the left of the decimal point. 2. It CAN be used whenever it's convenient. Often, this involves very small numbers. Summary It’s easy to keep track of significant figures if you remember WHY you’re doing it. WHY are you doing it? Significant figures reflect the accuracy of measured quantities and of results calculated from measured quantities.