CHAPTER #2
Measurement and Calculations
Measurement
• A measurement is a
quantitative observation.
• Measurements have 2 parts a
number and unit.
Number is a comparison
known found on a
measuring device.
Unit tells the type of
measurement and a scale.
Measurment
The number comes from a measuring device, such
as a ruler, clock, or speedometer, to name a few
examples of measuring devices. The unit is a word
or abbreviated word describing the measurement
and the scale used.
All measuring devices contain a scale. Scales
contain space between the lines. The last number of
a measurement, called a significant figure, is a
guess as to the number between the lines.
Measurements
Since measurements contain a guess, they cannot be exact.
Since measurements contain a guess, they cannot be exact.
11.64 cm
Measurements
11.64 cm
Since the last number is a guess most observers
would agree between 11.63-11.65 cm. This being the
case 11.64 is usually expressed as 11.64 ±0.01 cm
Measurements
When we make a measurement the last recorded
number is always an estimate due to reading
between the lines. If the object being measured
appears to be on the line, then a zero is used to
describe the fact that the object is on the line.
Measurements
This means that the last recorded number will
usually vary depending on who is estimating the last
number. This produces uncertainty, or error in the
measurement.
Significant Figure Definition
• Significant figures are the number of numbers
read from a measuring device.
What Are Numbers?
Numbers are any integers from 1- ∞, and
sometimes zero.
Zero serves two purposes, it is used as a
decimal place holder, a number, or both.
How do we determine if a zero is a number or
a position holder when determining the
number of significant figures for a
measurement?
The Zero Test
To determine if a zero is a number or a decimal spacer,
consider dropping one or more of the zero digits. If
dropping a zero changes the value of the measurement,
then the zero is a decimal position holder and is not
considered to be a number and therefore cannot be
counted as a significant figure.
For Example
Consider the measurement 100 cm. Dropping the
last two zeros changes the value to 1, so the zeros
are position holders and not numbers. Since
significant figures are numbers by definition, then
they are not counted in the significant figure count,
thus 100 cm has only one significant figure.
Now consider the measurement 100.0 cm. If the last
zero is dropped the value of the measurement
remains the same. Here the last zero does not
space the decimal in this measurement. Since zeros
are either decimal position holders, or numbers, then
the zero in this case must be a number and counted
in the significant figure count since is not a decimal
spacer.
Sandwiched Zeros
What about the zeros in the center of the
measurement of 100.0 cm? Since the last zero is
a number and the one at the beginning is a
number then the center zeros are sandwiched by
two numbers. Sandwiched zeros are always
counted as significant figures, thus giving 100.0
cm four significant figures
Zeros Both Numbers and Spacers?
For a zero to be counted as a spacer and a number
additional information must be given:
• Common sense to be gained in the laboratory
• A measuring device so states that they are
significant.
Examples
Consider the following list of measurements and determine
how many significant figures each measurement contains.
Measurements
10 cm
10.0 cm
101 cm
101.0 cm
1.00 X 10-3 cm
SigFigs
Examples
Consider the following list of measurements and determine
how many significant figures each measurement contains.
Measurements
10 cm
10.0 cm
101 cm
101.0 cm
1.00 X 10-3 cm
SigFigs
1
Reason
Zero is a spacer for sure. Additional information
required to see if it is a number
Examples
Consider the following list of measurements and determine
how many significant figures each measurement contains.
Measurements
SigFigs
Reason
10 cm
1
Zero is a spacer for sure. Additional information
required to see if it is a number
10.0 cm
3
The last number is not a spacer, since dropping
it the value is unchanged. The other zero is
sandwiched.
101 cm
101.0 cm
1.00 X 10-3 cm
Examples
Consider the following list of measurements and determine
how many significant figures each measurement contains.
Measurements
SigFigs
10 cm
1
10.0 cm
3
101 cm
3
101.0 cm
1.00 X 10-3 cm
Reason
Zero is a spacer for sure. Additional information
required to see if it is a number
Zero is sandwiched here
Examples
Consider the following list of measurements and determine
how many significant figures each measurement contains.
Measurements
SigFigs
10 cm
1
10.0 cm
3
101 cm
3
101.0 cm
4
1.00 X 10-3 cm
Reason
Zero is a spacer for sure. Additional information
required to see if it is a number
Zero is sandwiched here
Zero is not a spacer. The other zero is
sandwiched.
Examples
Consider the following list of measurements and determine
how many significant figures each measurement contains.
Measurements
SigFigs
10 cm
1
10.0 cm
3
101 cm
3
101.0 cm
4
1.00 X 10-3 cm
3
Reason
Zero is a spacer for sure. Additional information
required to see if it is a number
Zero is sandwiched here
The last zero is not a spacer . The other
zero is sandwiched.
Only look at the coefficient, these
zeros are not spacers
Example
How can we express 100 cm to three significant
figures?
Example
How can we express 100 cm to three significant
figures? Use Scientific Notation!
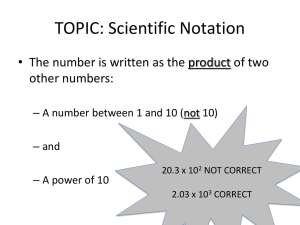
Scientific Notation
A way to abbreviate large or small numbers
1.
2.
3.
Place a decimal to the right of the
first nonzero number.
Place “X10” to the right of the
decimal number.
Count from the old decimal to the
new decimal. This number becomes
the power of 10; negative power, if
the number is less than one (if
number starts with zero, then it is
less than one)
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
4.54
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
4.54
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
4.54 X 10
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
Step 3, count from the old decimal location to the
new decimal location
4.54 X 10
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
Step 3, count from the old decimal location to the
new decimal location, this number of places
becomes the power of 10.
4.54 X 10
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
Step 3, count from the old decimal location to the
new decimal location, this number of places
becomes the power of 10.
4.54 X 105 mi
Scientific Notation Examples
Convert the following into scientific notation.
a. 454,000 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
Step 3, count from the old decimal location to the
new decimal location, this number of places
becomes the power of 10.
4.54 X 105 mi
Note: Be sure that the
answer contains the same
number of significant figures
as the starting measurement
Scientific Notation Examples
Convert the following into scientific notation.
b. 0.00283 mi
Step 1, place a decimal to the right of the first
non-zero number.
Scientific Notation Examples
Convert the following into scientific notation.
b. 0.00283 mi
Step 1, place a decimal to the right of the first
non-zero number.
2.83 mi
Note: Be sure that the answer contains
the same number of significant figures as
the starting measurement
Scientific Notation Examples
Convert the following into scientific notation.
b. 0.00283 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
2.83 X 10 mi
Note: Be sure that the answer contains
the same number of significant figures as
the starting measurement
Scientific Notation Examples
Convert the following into scientific notation.
b. 0.00283 mi
Step 1, place a decimal to the right of the first
non-zero number.
Step 2, place X 10 after the number.
Step 3, count from the old decimal location to the
new decimal location, this number of places
becomes the power of 10, unless the number is
less than one, if so, then negative power
Note: Be sure that the answer contains
2.83 X 10-3 mi the same number of significant figures as
the starting measurement
Example
How can we express 100 cm to three significant
figures? Use Scientific Notation!
Example
How can we express 100 cm to three significant
figures? Use Scientific Notation!
1.00 X 102
Review
1. If there are 37 students in this room, then how
many significant figures are there?
Review
1. If there are 37 students in this room, then how
many significant figures are there? None
Review
1. If there are 37 students in this room, then how
many significant figures are there? None
2. How many significant figures are there in 100
mL?
Review
1. If there are 37 students in this room, then how
many significant figures are there? None
2. How many significant figures are there in 100
mL? None
Review
1. If there are 37 students in this room, then how
many significant figures are there? None
2. How many significant figures are there in 100
mL? None
3. There are 5280 ft in a mile; how many significant
figures?
Review
1. If there are 37 students in this room, then how
many significant figures are there? None
2. How many significant figures are there in 100
mL? None
3. There are 5280 ft in a mile; how many significant
figures? None
Review
1. If there are 37 students in this room, then how
many significant figures are there? None
2. How many significant figures are there in 100
mL? None
3. There are 5280 ft in a mile; how many significant
figures? None
QUALITY OF MEASUREMNTS
• Accuracy-How close a measurement is to
the true value.
• Precision-How close multiple
measurements of the same objects are to
each other.
Examples of Accuracy and Precision
ROUNDING
When measurements are combined to provide information,
can the resultant information be of a higher quality than the
measurements?
ROUNDING
When measurements are combined to provide information,
can the information be of a higher quality than the
measurements? No, information provide by combining
measurements cannot have an accuracy, or precision
greater than the least precise measurement that provided
the information.
Why Round After a Calculation
Since information provided by combining measurements
cannot have a higher quality than the measurements
providing the information, then answers to problems must be
rounded to give the same quality as the measurement with
the least quality.
Rounding rules are designed to give answers the desired
quality. They are posted on the course web site and
restated on the following slide.
ROUNDING RULES
Rounding is the process of providing results that have the
same quality as measurements with the least quality. Since
there are different mathematical methods of combining
measurements, then different rounding rules are required to
provide sensible results of measurement combinations.
Addition and Subtraction
Round the calculated answer so that it contains the same
number of decimal places as the measurement with the
least number of decimal places.
Multiplication and Division
Round the calculated answer so that it contains the
same number of significant figures as the
measurement with the least number of significant
figures. In other words, if the measurement with the
least number of significant figures contains two
significant figures, then the rounded answer should
contain two significant figures.
Logarithms
Round the calculated answer so that it contains the
same number of decimal places as the
measurement with the least number of significant
figures. In other words, if the measurement with the
least number of significant figures contains two
significant figures, then the rounded answer should
contain two decimal places.
Anti-logarithms
Round answer so that the number of significant figures
matches the number of decimal places as the
measurement with the least number of decimal places. In
other words, if the measured number contains three decimal
places, then the answer should be rounded so that it contains
three significant figures.
SI Unit System
In chemistry we use the international system of units. This is
a modern version of the metric system.
Unfortunately this system of units is not widely used in
everyday life in the USA.
Being able to use conversion factors and formulas to
transform measurements between systems of units is
extremely important.
This procedure is called unit analysis
The Fundamental SI Units
SI means system international, or international system of
units
Physical Quantity
Mass
Name of Unit
kilogram
Abbreviation
kg
Length
Time
Temperature
meter
second
kelvin
m
s
K
Electric current
Amount of substance
ampere
mole
A
mol
Copyright © Cengage Learning. All rights reserved
Basic Metric Units
Some of the common units for measurements and their
abbreviations are shown below.
Measurement
Units
Abbreviation
Mass
grams
g
Volume
liters
L
Distance
meters
m
Time
seconds
s
A much more extensive table is given on page 24 of the text.
Common Metric Unit Prefixes
In chemistry we are often dealing with very large or
very small quantities. To help with this a system of
prefix modifiers have been developed. Please
Memorize this list. (Note a more extensive list is on page
26 of your text)
Prefix
Abbreviation
Multiplier
mega
M
1000 000 (106)
kilo
k
1000 (103)
deci
centi
d
c
0.1 (10-1)
0.01 (10-2)
milli
micro
m
μ
0.001 (10-3)
0.000001 (10-6)
Application of Metric Prefixes
Length (m)
Mass (g)
Time (s)
103 m = km
103 g = kg
103 s = ks
10-2 m = cm
10-2 g = cg
10-2 s = cs
10-3 m = mm
10-3 g = mg
10-3 s = ms
10-6 m = µm
10-6 g = µg
10-6 s= µs
Note: The memorized number always is in front
of the single letter.
Unit Conversion Accidents
There have been many serious incidents that have resulted from
errors in converting between systems of units.
Air Canada Flight 143
(Google it for more details)
Unit Conversion Accidents
$125 million Mars Climate Orbiter.
Lost in Space.
Dp you think there is the potential to make errors in the
conversion of units for health care providers?
Conversion Problem Steps
1. Write down the number and unit.
2. Draw lines; a vertical line after the number an
unit and horizontal line below the number and
unit.
3. Insert a fractional fact to cancel out the
original unit.
4. Compare the new unit to the asked for unit
a. If the same, you are done.
b. If not the same, repeat step 3.
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
47.2 mg
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
47.2 mg
Step 2. Draw lines
47.2 mg
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
47.2 mg
Step 2. Draw lines
47.2 mg
Step 3. Insert fractional fact crossing out original unit
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
47.2 mg
Step 2. Draw lines
47.2 mg
Step 3. Insert fractional fact crossing out original unit
47.2 mg 10-3 g
mg
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
47.2 mg
Step 2. Draw lines
47.2 mg
Step 3. Insert fractional fact crossing out original unit
47.2 mg 10-3 g
mg
Step 4. Compare new unit to the asked for unit.
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
47.2 mg
Step 2. Draw lines
47.2 mg
Step 3. Insert fractional fact crossing out original unit
47.2 mg 10-3 g
mg
Step 4. Compare new unit to the asked for unit.
A. If the same you are done
b. If not the same repeat step 3.
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
47.2 mg
Step 2. Draw lines
47.2 mg
Step 3. Insert fractional fact crossing out original unit
47.2 mg 10-3 g
= 0.0472 g
mg
Step 4. Compare new unit to the asked for unit.
A. If the same you are done
b. If not the same repeat step 3.
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL.
Step 1. Write down the number and unit.
702 cL
Step 2. Draw lines
702 cL
Step 3. Insert fractional fact crossing out original unit
10-2 L
cL
Step 4. Compare new unit to the asked for unit.
A. If the same you are done
b. If not the same repeat step 3.
702 cL
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL. Not a match repeat step #3
Step 1. Write down the number and unit.
702 cL
Step 2. Draw lines
702 cL
Step 3. Insert fractional fact crossing out original unit
10-2 L
cL
Step 4. Compare new unit to the asked for unit.
A. If the same you are done
b. If not the same repeat step 3.
702 cL
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL. It’s a match, done
Step 1. Write down the number and unit.
702 cL
Step 2. Draw lines
702 cL
Step 3. Insert fractional fact crossing out original unit
10-2 L μL
cL
10-6 L
Step 4. Compare new unit to the asked for unit.
A. If the same you are done
b. If not the same repeat step 3.
702 cL
Sample Conversion Problems
1. How many grams are in 47.2 mg?
2. Change 702 cL to µL. It’s a match, done
Step 1. Write down the number and unit.
702 cL
Step 2. Draw lines
702 cL
Step 3. Insert fractional fact crossing out original unit
702 cL
10-2 L μL
cL
10-6 L
= 7.02 x 106 μL
Step 4. Compare new unit to the asked for unit.
A. If the same you are done
b. If not the same repeat step 3.
DENSITY
• What is heavier 5 pounds of lead or 5
pounds of feathers?
• What takes up more space, 5 pounds of lead
or 5 pounds of feathers?
DENSITY
• What is heavier 5 pounds of lead or 5
pounds of feathers? Both the same. This
is an old riddle to confuse density with
weight
• What takes up more space, 5 pounds of lead
or 5 pounds of feathers?
DENSITY
• What is heavier 5 pounds of lead or 5
pounds of feathers? Both the same. This
is an old riddle to confuse density with
weight
• What takes up more space, 5 pounds of lead
or 5 pounds of feathers? Feathers, since
they are less dense.
Archimedes Principle
We can determine the volume of irregularly shaped objects by
displacement.
How can we determine the volume of a gas?
Gases fill whatever container they are placed in. So it’s the
volume of the container !
DENSITY UNITS
g/ml, g/cm3, (for solids and liquids), or
g/L for gases
DENSITY PROBLEM SOLVING STRATEGY
Use the four step unit analysis. Organize the
measurements to give density units.
Sample Problems
1. Calculate the density of a 4.07 g sample of rock that
displaces 1.22 mL of water.
2. Calculate the density of a 4.22 g sample of wood that
measures 2.0 cm by 1.33 cm by 3.56 cm.
3. Mercury has a density of 13.6 g/mL. Find the mass of
125 mL of mercury.
4. Water has a density of 1.00 g/mL. Find the volume, in
liters, of a 3.22 kg sample of water.
5. What does an object do in water with
a. A density greater than water?
b. A density less than water?
c. A density equal to water?
English/Metric Conversions
Definitions
2.54 cm = in
946 ml = qt
454 g = lb
cm3 = mL
Please Remember
Definitions are not
measurements and do not
contain significant figures
Sample English/Metric Conversion Problems
1. Convert 708 pounds to kilograms.
2. Convert 50.0 liters to gallons.
3. Convert the density of water to pounds per gallon.
4. How many cubic meters are contained in 33 liters?
5. The density of aluminum is 2.70 g/mL. Find the
thickness of aluminum foil that measures 2.0 cm by
5.66 cm that has a mass of 1.23 g.
The End