Shape
advertisement
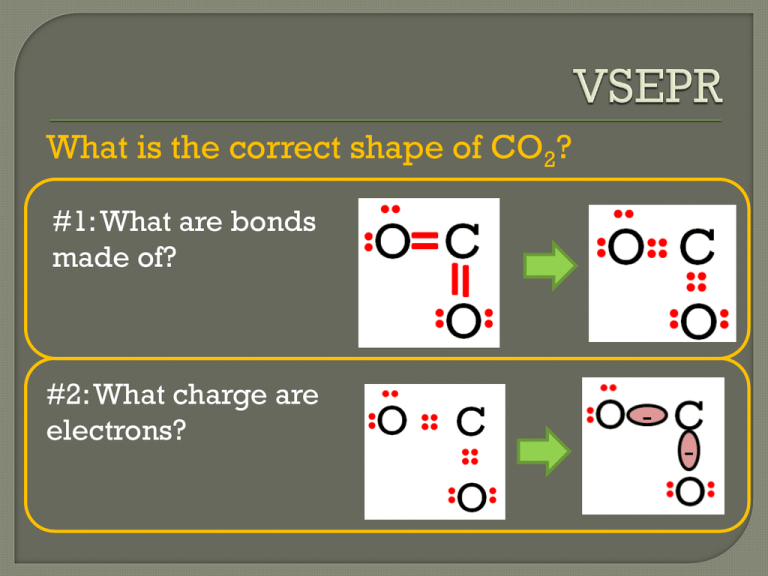
What is the correct shape of CO2? #1: What are bonds made of? #2: What charge are electrons? What is the correct shape of CO2? Will those (-) charges attract or repel? (Clue: look at 2 balloons) A) Attract B) Repel Question: Key Pt 1: Bonding Groups Single bonds = 1 bonding group Double bonds = Triple bonds Example: CCl4 - Bonding Groups = 4 Question: Key Pt 1: Bonding Groups Single bonds = 1 bonding group Double bonds = 1 bonding group Triple bonds Example: CHOF - Bonding Groups= 3 Question: Key Pt 1: Bonding Groups Single bonds = 1 bonding group Double bonds = 1 bonding group Triple bonds 1 bonding group Example: CHOF - Bonding Groups= 2 Key Pt 1: Bonding Groups Question: Single lone pair= 1 nonbonding group Two lone pairs= Three lone pairs = Example: AsH3 -Nonbonding Groups= 1 Key Pt 2: Nonbonding Groups Question: Single lone pair= 1 nonbonding group Two lone pairs= 2 nonbonding group Three lone pairs = 3 nonbonding group Example: SHCl -Nonbonding Groups= 2 Key Pt 2: Nonbonding Groups Question: Single lone pair= 1 nonbonding group Two lone pairs= 2 nonbonding group Three lone pairs = 3 nonbonding group Example: SHCl -Nonbonding Groups= 2 Key Pt 2: Nonbonding Groups Question: Single lone pair= 1 nonbonding group Two lone pairs= 2 nonbonding group Three lone pairs = 3 nonbonding group Example: SHCl -Nonbonding Groups= 0 How many bonding groups are there? (Double Bonds = Just one bonding group) A) 3 B) 4 C) 8 How many nonbonding groups are there? (Nonbonding: Only count around central atom) A) 0 B) 3 C) 5 32 0 How many nonbonding groups are there? (Nonbonding: Only count around central atom) A) 0 B) 3 C) 5 Cut and paste into your notebooks. Question: alence hell lectron air epulsion spread out as much as possible. A) Attract B) Repel alence hell lectron air epulsion spread out as much as possible. Step 1: Molecule Count # Electron Groups around central atom 2 bonding groups Step 2: Balloon Spread out as far as possible. Angle? 180° Step 3: Geometry Determine the shape. Linear A)Linear B) Trigonal Planar C)Trigonal Pyramid D)Tetrahedral VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups 2 Electron Groups Electron Groups 2 Bonding Groups Shape: Linear Shape: Shape: Shape: Shape: alence hell lectron air epulsion spread out as much as possible. Step 1: Molecule Count # Electron Groups around central atom 3 bonding groups Step 2: Balloon Spread out as far as possible. Angle? 120° Step 3: Geometry Determine the shape. Trigonal Planar A)Linear B) Trigonal Plana C)Tetrahedral VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups Electron Groups 2 Bonding Groups 3 Bonding Groups 2 Electron Groups 3 Shape: Shape: Linear Trigonal Planar Shape: Shape: Shape: How will these groups spread out? (How many electron groups? 4 electron groups) (Balloon demo: what happens?) A) B) C) None of the above What shape is this most like? (How many corners? 4 corners) A) Triangular Pyramid B) Rectangular Pyramid What else is this shape called? (Mono = 1, Di = 2, Tri = 3, Tetra = 4, Penta = 5) A) Trihedron B) Quadrahedron C) Tetrahedron alence hell lectron air epulsion spread out as much as possible. Step 1: Molecule Count # Electron Groups around central atom 4 bonding groups Step 2: Balloon Spread out as far as possible. Angle? 109.5° Step 3: Geometry Determine the shape. Tetrahedral A)Linear B) Trigonal Plana C)Tetrahedral VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups Electron Groups 2 Bonding Groups 3 Bonding Groups 2 Electron Groups 3 4 4 Bonding Groups Shape: Shape: Shape: Linear Trigonal Planar Tetrahedral Shape: Shape: What kind of shape will form? (Bonding groups = 3) A) Trigonal Planar C)Tetrahedral B) Trigonal Pyramidal VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups Electron Groups 2 Bonding Groups 3 Bonding Groups 2 Electron Groups 3 4 4 Bonding Groups Shape: Shape: Shape: Linear Trigonal Planar Tetrahedral Shape: Shape: What kind of shape will form? (Bonding groups = 3) A) Trigonal Planar C)Tetrahedral B) Trigonal Pyramidal What kind of shape will form? (Bonding groups = 2) A) Trigonal Planar C)Tetrahedral B) Trigonal Pyramidal VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups Electron Groups 2 Bonding Groups 3 Bonding Groups 2 Electron Groups 3 4 4 Bonding Groups Shape: Shape: Shape: Linear Trigonal Planar Tetrahedral Shape: Shape: What kind of shape will form? (Bonding groups = 3) A) Tetrahedral C) Linear B) Straight alence hell lectron air epulsion spread out as much as possible. Step 1: Molecule 3 bonding, Count # Electron Groups 1 nonbonding around central atom Step 2: Balloon Spread out as far as possible. Angle? Step 3: Geometry Determine the shape. 109.5° 109.5° Trigonal Pyramidal VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups 2 2 Bonding Groups Electron Groups Electron Groups 3 3 Bonding Groups 4 4 Bonding Groups Shape: Shape: Shape: Linear Trigonal Planar Tetrahedral 3 bonding 1 nonbonding Shape: Trigonal Pyramidal Shape: What will the overall shape of SF2 be? (Count around central atom only) A) Linear B) Tetrahedral How many corners will SF2 have? (1 atom = 1 corner) A) 2 corners B) 3 corners C) 4 corners Remove one more dot from candy tetrahedron. What letter does it look like now? V-shaped alence hell lectron air epulsion spread out as much as possible. Step 1: Molecule 2 bonding, Count # Electron Groups 2 nonbonding around central atom Step 2: Balloon Spread out as far as possible. Angle? 109.5° Step 3: Geometry Determine the shape. Bent 109.5° VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups 2 2 Bonding Groups Electron Groups Electron Groups 3 3 Bonding Groups 4 4 Bonding Groups Shape: Shape: Shape: Linear Trigonal Planar Tetrahedral 3 bonding 2 bonding 1 nonbonding 2 nonbonding Shape: Trigonal Pyramidal Shape: Bent What is the shape of PCl3? (# Bonding = 3 # Nonbonding = 1 A) Trigonal Planar B) Trigonal Pyramidal C) Tetrahedral ) VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups 2 2 Bonding Groups Electron Groups Electron Groups 3 3 Bonding Groups 4 4 Bonding Groups Shape: Shape: Shape: Linear Trigonal Planar Tetrahedral 3 bonding 2 bonding 1 nonbonding 2 nonbonding Shape: Trigonal Pyramidal Shape: Bent What is the shape of PCl3? (# Bonding = 3 # Nonbonding = 1 A) Trigonal Planar B) Trigonal Pyramidal C) Tetrahedral ) What is the shape of SCl2? (# Bonding = 2 # Nonbonding = 2 A) Linear B) Tetrahedral C) Bent ) VSEPR:Valence Shell Electron Pair Repulsion. Electron Groups stay as far apart as possible. Electron Groups 2 2 Bonding Groups Electron Groups Electron Groups 3 3 Bonding Groups 4 4 Bonding Groups Shape: Shape: Shape: Linear Trigonal Planar Tetrahedral 3 bonding 2 bonding 1 nonbonding 2 nonbonding Shape: Trigonal Pyramidal Shape: Bent What is the shape of PCl3? (# Bonding = 2 # Nonbonding = 2 A) Linear B) Tetrahedral C) Bent )


