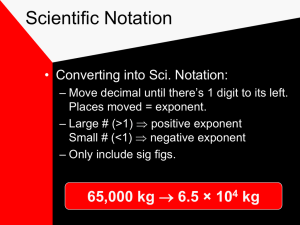
Measurement: Accuracy, Precision, Metrics & Sig Figs
advertisement

K (what do you know About Measurement) W (what do L (what you want to have you know learned) about Measurement In the context of the scientific method, precision and accuracy have two distinctly different meanings. The accuracy of a measurement system is the degree of closeness of measurements of a quantity to its actual (true)value. The precision of a measurement system is the degree to which repeated measurements under unchanged conditions show the same results. See dartboard (p. 44) A measurement system can be accurate but not precise, precise but not accurate, neither, or both. www.yorku.ca/psycho/en/pics_en/postscript_f1.gif % error = measured – accepted accepted X 100 p. 45 #1 practice problem Find the % error if a mass measurement is 17.7 grams when the correct mass is 21.2 grams. A liquid’s volume is measured in a beaker as 40 mL. It is then measured in a graduated cylinder as 45.5 mL. Find the % error. P. 45 # 1—2 p. 60 #35—37 1. 2. 3. 4. 5. 6. 7. 8. 0.9% 0.4% 3.5% 9.3% 1.7% 3.7% 0.3% 10.4% MEASUREMENT WARM UP 1. Based on the following data collected, comment on this person’s accuracy & precision: ***Volume in beaker trial 1= 30.0 mL Trial 2 = 31.0 Trial 3= 31. 5 ***Volume in cylinder = 45.5 mL 2. Calculate % error: A student measures mass as 50.9 grams. The instructor measures 55.9 grams. 1. high precision; low accuracy 2. 8.9% Write a paragraph to your friend explaining to him or her the difference between accuracy vs. precision. Include an example using the dartboard analogy. (refer to p. 44 if needed) CALCULATE % ERROR FOR THE FOLLOWING: 1. A student measures the volume of a cube to be 20.5 cubic centimeters. He checks this against the correct volume which is 25 cubic centimeters. 2. A liquid’s volume is 35 mL in a graduated cylinder, while in a beaker the volume is 25 mL. 3. Comment On precision And accuracy For picture D. http://www.brookscole.com/math_d/sp ecial_features/ext/internet_activities/ matovina/metric ONLY COMPLETE 1—6 and #8 TODAY USE YOUR OWN NOTEBOOK PAPER Why is the metric system of measurement (which uses meters, liters, grams, etc.) preferred AND easier to use rather than the English system of measurement (which uses pounds, feet, etc.)? 1 1 1 1 1 foot. = 12 inches pound = 16 ounces cup = 8 ounces yard = 3 feet mile = 5280 feet Also known as the SI based system (International System of Measurements) It is more preferred rather than the English system of measurements because it is based on units of 10. Measurement systems are all based on “standards” which are physical representations for each measurement unit. We will learn about the prefixes “Tera” through “pico”. (see chart) Mass = gram Distance = meter Volume = liters or cubic meter Time = seconds Temperature = Kelvin Amount of a substance = mole Electric current = ampere Light intensity = candela Area = square meter Energy = joule 1 KILOmeter = ________________meters 1 CENTIgram = ______________________grams 1 millisecond = __________________seconds THE GREAT MIGHTY king henry died by drinking chocolate milk maybe not pasteurized mg kL Mm mm um m Name 3 basic metric units. Name 2 prefixes AND give their symbol. Name 1 thing you know about a “standard” of measurement. 1. liquids—graduated cylinder (measures in mL) 2. rectangular shaped solids- use a ruler for length x width x height (measured in cubic cm or cm3 or cc) 3. Irregular shaped solids—water displacement ***VOLUME—the amount of space something takes up 25 mL = ___________________cc (cm3) 25 cc = ________________________L 1—12, 15, 16 Describe how you would take the volume of a glass of water, a rectangular block, and a marble. Also, tell what units that you would use to measure each. WRITING ASSIGNMENT: (1-2 paragraphs) Discuss the differences between area & volume. Include in your discussion: ways they are measured, units which they are measured in, and tools used to measure them. Also, remember the different methods for measuring volume. % ERROR/METRICS 1. A lab tech measures the boiling point of water to be 99.5 C. The true boiling pt of water is 100.0 C. Calculate the % error. (SHOW WORK!!) 2. 0.0075 g = ______________________ng 3. 3400 kg = ____________________Mg 4. 258 daL = _______________________mL 5. 534 L = __________________cm3 1. 2. 3. 4. 5. 99.5 – 100.0 x 100 = 0.5% 100.0 7 500 000 ng 3.4 Mg 2 580 000 mL 534 000 cc (mL) In a paragraph, describe how mass, volume, length, and temperature are measured. PART III #8 SHOW WORK FOR % ERROR Accepted values: Bottle = 7.095 grams Clamp = 75.069 grams Domino = 5.371 grams Stopper = 7.090 grams METRIC WARMUP GIVE THE SYMBOL FOR EACH UNIT: 1. Micrometer 2. Meter 3. Kiloliter 4. Megameter 5. millimeter WHAT QUANTITY DO THESE UNITS MEASURE? 7. meter 8. cc 9. liter 10. gram 11. Cm3 12. Square meter PERFORM THESE METRIC CONVERSIONS: 13. 14. 15. 16. 2.67 ng = _______________pg 34000 m = ______________Mm 50 cc =_______________mL 3 L = __________________cm3 This is done when BOTH units have the SAME exponent (squared to squared or cubed to cubed) Ex: 100 cm2 = _______________m2 cm to m is normally 2 spaces left, so multiply this 2 spaces x the exponent of 2 = total of 4 spaces left Ex: 0.0075 Mm3 = ________________km3 Mm to km is normally 3 spaces right, so multiply by exponent of 3 = 9 spaces right 1. 55 cc = ______L 2. 0.00035 Mm = _________m 3. 675 dL =____________kL 4. What do millimeters measure? 5. What do kilograms measure? 6. What do liters measure? 7. What do cubic millimeters measure? 8. What do square centimeters measure? 9. Which is larger: 250 cc or 0.5 L? 10. Find the area of a box measuring 5 cm by 8 cm. 11. Does milli make the base unit larger or smaller? 12. Find the volume of a rock that’s dropped in 25 mL of water and the level then rises to 38 mL. 1. 0.055 L 2. 350 m 3. 0.0675 kL 4. length 5. mass 6. volume 7. volume 8. area 9. 0.25 L or 0.5 L 10. 5 cm x 8 cm = 40 cm2 11. smaller 12. 38 – 25 = 13 mL BOOK PROBLEMS (% error, metrics, accuracy/precision) p. 59 #1, 4, 7, 8 ab, 9 ab, 16, 20, 21, 24, 25, 37 p. 63 #1, 3, 4, 6, 7, 8 Explain why error always exists in measurement. Significant figures help scientists be able to do the same thing when taking measurements and doing calculations. SEE P. 46 1. 2. 3. 4. Digits from 1-9 are always significant. Zeros between two other significant digits are always significant Final zeros to the right of the decimal place are significant. Zeros used solely for spacing the decimal point (placeholders) are not significant (Unless specifically measured and noted with a line above the number). The letters "A" (decimal absent) and "P" (decimal present) correspond to the "Atlantic" and "Pacific" Oceans on a map. Now, imagine an arrow being drawn from the appropriate coast. Once the arrow hits a NONZERO digit, this digit and all of the digits after it are significant. Example 1. How many significant digits are shown in the number 20 400 ? Well, there is no decimal, so we think of "A" for "Absent". This means that we imagine an arrow coming in from the Atlantic ocean 20 400 this shows 3 significant numbers as you do not count numbers until you hit a significant digit Modified from http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson23.htm Example 2. How many significant digits are shown in the number 0.090 ? Well, there is a decimal, so we think of "P" for "Present". This means that we imagine an arrow coming in from the Pacific ocean. 0.090 This shows that the number has two significant digits after the non zero number is encountered Modified from http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson23.htm 1. 2. 3. 4. 5. 6. 7. 8. 2300. m 2300 g 0.005 L 23.92 sec 40,060 kg 2005 moles 32.00 Kelvin 43.090 Mm 1. 2. 3. 4. 5. 6. 7. 8. P=4 A= 2 P=1 P=4 A=4 A=4 P=4 P=5 TELL IF THE FOLLOWING ARE “ATLANTIC” OR “PACIFIC” AND THEN TELL HOW MANY SIG FIGS: 1. 0.0035 cm 7. 0.004 mg 2. 10.00 g 8. 549000 cm 3. 3400 m 9. 3000 g 4. 53.57 mm 10. 0.45670 nm 5. 40600 kg 11. 2734 km 6. 200.040 Mm 12. 5.070 sec TELL IF THE FOLLOWING ARE “ATLANTIC” OR “PACIFIC” AND THEN TELL HOW MANY SIG FIGS: 1. 67. 930 g 2. 3. 4. 5. 6. 7. 8. 9. 10. 2600 m 0.0070 km 5030 cm 67.00 mm 3.69 sec 0.03 mm 1000 kg 1000. Mm 63.500 kg You may ONLY do this to zeros in ATLANTIC numbers 1. put a bar over the zero 2500—has 2 sig figs How to make 3 sig figs?? How to make 4 sig figs?? Put a decimal at the end (making it a pacific #) 2500 2500. 2. TELL HOW MANY SIG FIGS (1ST DECIDE IF A or P) 1. 21.34 g 2. 52.340 g 3. 28,007 L 4. 80.00 m 5. 0.0025 g 6. 23,000 cm 7. 28, 875 mm 8. 505,100 g 9. 0.050 L IN EACH: 10. 11. 12. 13. 14. 15. 16. 17. 18. 51.200 g 6050 m 2000 L 40.50 cm 0.192 m 3000. L 30 mm 30,650 Mm 0.00500 g 1. 4 2. 5 3. 5 4. 4 5. 2 6. 5 7. 5 8. 5 9. 2 10. 5 11. 3 12. When trying to do this, move through the number from left to right. Ex: Round to 1 sig fig: 2300 0.0897 5.9 Round to 2 sig figs: 2895 0.0956 Round to 3 sig figs: 2895 0.6 TELL HOW MANY SIG FIGS ARE IN EACH--1st decide if A or P: 1. 0.00306 5. 3000. 2. 0.003060 6. 43.06 3. 4300 7. 3.020 4. 4060 8. 5000 ROUND EACH TO 2 SIG FIGS: 1. 0.0357 4. 657 2. 2350 5. 0.0695 3. 90.34 6. 0.7 ROUND EACH TO 1 SIG FIG: 1. 369 3. 0.0078 2. 20.47 4. 379.5 9. 2.0 1. 2. 3. 4. 3 4 2 4 1. 0.036 2. 2400 3. 90. 1. 2. 3. 4. 400 20 0.008 400 5. 6. 7. 8. 4 4 4 1 4. 660 5. 0.070 6. 0.70 9. 2 Round answer to the FEWEST DECIMAL PLACES that are in the problem 10.711 g +3.23 g 4 mL -3.4 mL 5.75 cm +2.976 cm 1. 2. 3. 13.941 = round to 2 dec. = 13.94 g 0.6 = round to 0 dec. = 1 mL 8.726 = round to 2 dec. = 8.73 cm answer to the FEWEST SIG FIGS that are in the problem Round 2.32 cm x 77.96 cm = 62.0 g / 2.000 mL = 1.805 m x 6.0 m = 1. 2. 3. 180.8672=round to 3 sig figs = 181 cm2 31=round to 3 sig figs = 31.0 g/mL 10.83= round to 2 sig figs = 11m2 Give any 3 measurements and tell how many sig figs are in each. Describe the 2 different ways to round (add/subtract VS. Multiply/divide) Name 1 way to make zeros significant when they’re not to begin with. 1—34 EVEN 22. 30.647 = 30.6 grams 24. 9.2946 = 9.29 L 26. 29.56 = 30 sec 28. 1.967 = 2 g/mL 30. 0.022737 = 0.023 sq inches 32. 0.25 = 0. 250 kg/L 34. 0.012049 = 0.01205 Mm2 1. 2. 3. 4. 5. 5.0 m X 457 m= 16.56 g + 13 g = 5.60 g / 22.4 L = 0.059 g / 0.03 L = 14.26 cm - 4.9654 cm = 2285 = round to 2 sig figs = 2300 m2 29.56 = round to no decimal places = 30 g 0.25 = round to 3 sig figs = 0.250 g/L 1.966666 = round to 1 sig fig = 2 g/mL 9.2946 =round to 2decimal places = 9.29 cm P. 50 #1—3 P. 57 #3—4 Why would someone want to put a measurement into scientific notation? Reduces the number of zeros in really big or really small numbers The number in front determines the number of sig figs Starting out, the decimal MUST be written to the right of the first nonzero number in order to be in correct scientific form; then, depending on the exponent, it can be moved left or right to convert to a regular number. 5.64 x 104 (correct form with 3 sig figs) 0.0035 x 102 (incorrect form) Why?? 340 x 103 (incorrect form) Why?? 6.023 x 1023 4 sig figs 6.67 x 10-11 3 sig figs 2.00 x 10-3 3 sig figs In your calculator, 3.05 x 109 may appear: 3.05 3.05 3.05 E9 EE 9 9 If the exponent is POSITIVE, move decimal that many places to the RIGHT. Ex: 3.450 x 103 ***Remember to keep SIG FIGS the same!! If the exponent is NEGATIVE, move decimal that many places to the LEFT. Ex: 6.090 x 10-3 ***Remember to keep SIG FIGS the same!! 1. 4.560 x 105 = 2. 3.9 x 10-3 = 3. 5.0 x 100 = Remember that the decimal must be moved to the right of the first nonzero digit. Also, remember to keep SIG FIGS the same. If the number is greater than 1 to start with, use a positive exponent. Ex: 305,000 If the number is less than 1 to start with, use a negative exponent. Ex:0.004060 1. 456,000. = 2. 0.003400 = 3. 67000 = SIG FIGS---EVEN (OPTIONAL) SCIENTIFIC NOTATION--EVEN WARM UP PUT INTO SCIENTIFIC NOTATION: 1. 0.0060 2. 23500 PUT INTO STANDARD FORM (REGULAR NUMBER) 3. 3.50 X 102 4. 6.788 X 10-3 P. 57 #6 P. 60 #43—45 p. p. p. p. p. p. 48 57 60 31 42 59 #1-2 #1-3 #29, 38 #2 #1-3 #6-9, 16, 20, 21, 23, 25a, 38, 50, 51 WHAT TO STUDY FOR MEASUREMENT TEST 1. Qualitative vs. quantitative measurement 2. What’s the purpose of a standard in measurement 3. Basic units for length, time, volume, mass, and temp. 4. Metric prefix symbols, numerical meanings, and exponent meanings 5. Metric conversions (normal, cc = mL, and exponent ones) 6. Units for area and volume 7. 3 ways to measure volume 8. % error 9. Accuracy vs. precision 10. Mass vs. weight 11. Counting # of sig figs AND calculation rules for sig figs 12. Scientific notation into standard form (and vice-versa) 13. Vocabulary sheet 14. Reading metric tools 15. Measurement video ?s