Coulomb`s Law
advertisement
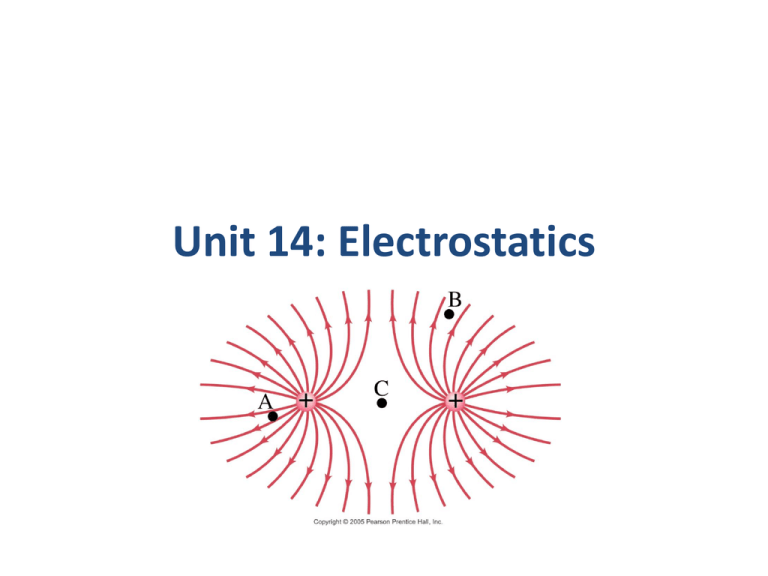
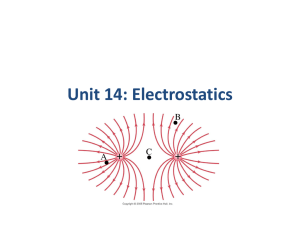
Unit 14: Electrostatics Units of Chapter 16 • Static Electricity; Electric Charge and Its Conservation • Electric Charge in the Atom • Insulators and Conductors • Induced Charge; the Electroscope • Coulomb’s Law • Solving Problems Involving Coulomb’s Law and Vectors • The Electric Field Units of Chapter 16 • Field Lines • Electric Fields and Conductors Do Now 1. How do protons and electrons differ in their electrical charge? 2. Is an electron in a hydrogen atom the same as an electron in a uranium atom? 3. Which has more mass – a proton or an electron? 4. In a normal atom, how many electrons are there compared to protons? Do Now 1. How do protons and electrons differ in their electrical charge? Same magnitude, but opposite charge. 2. Is an electron in a hydrogen atom the same as an electron in a uranium atom? Yes. 3. Which has more mass – a proton or an electron? Proton 4. In a normal atom, how many electrons are there compared to protons? Same number, no net charge. Atomic Structure 16.1 Static Electricity; Electric Charge and Its Conservation Objects can be charged by rubbing Triboelectric Series • Friction can cause electrons to transfer from one material to another • Different materials have a different degree of attraction for electrons • The triboelectric series determines which materials have a greater attraction. • When two materials are rubbed together, the one with the higher attraction will end up getting some of the electrons from the other material Triboelectric Series If two materials are rubbed together, the one that is higher in the series will give up electrons and become more positive. MORE POSITIVE Human Hands (if very dry) Leather Rabbit Fur Glass Human Hair Nylon Wool Fur Lead Silk Aluminum Paper Cotton Steel (neutral) Wood Amber Hard Rubber Nickel, Copper Brass, Silver Gold, Platinum Polyester Styrene (Styrofoam) Saran Wrap Polyurethane Polyethylene (scotch tape) Polypropylene Vinyl (PVC) Silicon Teflon MORE NEGATIVE Question • If fur is rubbed on glass, will the glass become positively charged or negatively charged? • Glass – positive • Fur -negative 16.1 Static Electricity; Electric Charge and Its Conservation Charge comes in two types, positive and negative; like charges repel and opposite charges attract Interaction Between Charged and Neutral Objects • Any charged object - whether positively charged or negatively charged - will have an attractive interaction with a neutral object. 16.1 Static Electricity; Electric Charge and Its Conservation Electric charge is conserved – the arithmetic sum of the total charge cannot change in any interaction. 16.2 Electric Charge in the Atom Atom: Nucleus (small, massive, positive charge) Electron cloud (large, very low density, negative charge) 16.2 Electric Charge in the Atom Atom is electrically neutral. Rubbing charges objects by moving electrons from one to the other. 16.2 Electric Charge in the Atom Polar molecule: neutral overall, but charge not evenly distributed 16.3 Insulators and Conductors Conductor: Insulator: Charge flows freely Almost no charge flows Metals Most other materials Some materials are semiconductors. How Charge Is Transferred • Objects can be charged by rubbing • Metal objects can be charged by conduction • Metal objects can be charged by • induction 16.4 Induced Charge; the Electroscope Metal objects can be charged by conduction: Charging by Induction • When an object gets charged by induction, a charge is created by the influence of a charged object but not by contact with a charged object. The word induction means to influence without contact. • If a rubber balloon is charged negatively (perhaps by rubbing it with animal fur) and brought near (without touching) the spheres, electrons within the two-sphere system will be induced to move away from the balloon. Charging By Induction With Negatively Charged Object In step iii, why is the charge on the right sphere almost uniformly distributed? Charging By Induction With Negatively Charged Object What was the source of positive charge that ended up on sphere B? Source of charge in induction • In induction, the source of charge that is on the final object is not the result of movement from the charged object to the neutral object. Ground • An infinite source or sink for charge 16.4 Induced Charge; the Electroscope They can also be charged by induction: 16.4 Induced Charge; the Electroscope The electroscope can be used for detecting charge: 16.4 Induced Charge; the Electroscope The electroscope can be charged either by conduction or by induction. 16.4 Induced Charge; the Electroscope The charged electroscope can then be used to determine the sign of an unknown charge. 16.4 Induced Charge; the Electroscope Nonconductors won’t become charged by conduction or induction, but will experience charge rearrangement. The atoms or molecules become polarized. : 16.4 Induced Charge; the Electroscope Nonconductors won’t become charged by conduction or induction, but will experience charge separation: Do Now True or False? Explain your reasoning. 1. An object that is positively charged contains all protons and no electrons. False Positively charged objects have electrons; they simply possess more protons than electrons. 2. An object that is electrically neutral contains only neutrons. False Electrically neutral atoms simply possess the same number of electrons as protons. Coulomb’s Law • The French physicist Charles Coulomb (1736 – 1806) investigated electric forces in the 1780s using a torsion balance. 16.5 Coulomb’s Law Experiment shows that the electric force between two charges is proportional to the product of the charges and inversely proportional to the distance between them. Coulomb’s Law 1. If two point charges Q1 and Q2 are a distance r apart, the charges exert forces on each object of magnitude: Q1 Q2 F1on 2 F2 on1 k 2 r These forces are an action/reaction pair, equal in magnitude but opposite in direction. 16.5 Coulomb’s Law 2. The forces are along the line connecting the charges, and is attractive if the charges are opposite, and repulsive if they are the same. 16.5 Coulomb’s Law Unit of charge: coulomb, C The proportionality constant in Coulomb’s law is then: When we only need two significant figures: k 9.0 10 N m / C 9 2 2 Charges produced by rubbing are typically around a microcoulomb: 16.5 Coulomb’s Law Charge on the electron: Electric charge is quantized in units of the electron charge. This is the smallest charge in nature – fundamental or elementary charge. The net charge of any object must be a multiple of that charge. 16.5 Coulomb’s Law The proportionality constant k can also be written in terms of , the permittivity of free space: (16-2) 16.5 Coulomb’s Law Coulomb’s law strictly applies only to point charges. Superposition: for multiple point charges, the forces on each charge from every other charge can be calculated and then added as vectors. 16.6 Solving Problems Involving Coulomb’s Law and Vectors The net force on a charge is the vector sum of all the forces acting on it. 16.6 Solving Problems Involving Coulomb’s Law and Vectors Vector addition review: Example 1 Suppose that two point charges, each with a charge of +1.00 Coulomb are separated by a distance of 1.00 meter. Determine the magnitude of the electrical force of repulsion between them. Given: Find: F - ? IQI I= 1.00 C Q2 I= 1.00 C r = 1.00 m Solve: F k Q1 Q2 r 2 (9.0 10 )(1)(1) 9 F 2 1 9.0 10 N 9 The force of repulsion of two +1.00 Coulomb charges held 1.00 meter apart is 9 billion Newton. This is an incredibly large force that compares in magnitude to the weight of more than 2000 jetliners. Example 2 Determine the magnitude and direction of the electric force on the electron of a hydrogen atom exerted by the single proton that is the atom’s nucleus. Assume the average distance between the revolving 10 electron an the proton r 0.53 10 m Given: Find: F-? Q1 Q2 1.6 10 19 C r 0.531010 m Solution: F k Q1 Q2 r2 (9.0 109 N m 2 / C 2 )(1.6 10 19 C )(1.6 10 19 C ) 8 8 . 2 10 N 10 2 (0.53 10 m) Problem 1 A balloon with a charge of 4.0 10 5 C is held a distance of 0.10m from a second balloon having the same charge. Calculate the magnitude of the electrical force between the charges. Draw a diagram. F k Q1 Q2 r2 (9.0 109 N m 2 / C 2 )(4.0 10 5 C )(4.0 10 5 C ) 1 14400 10 1440 N 2 (0.10m) Problem 2 • Calculate the electrical force exerted between a 22-gram balloon with a charge of -2.6μC and a wool sweater with a charge of +3.8μC; the separation distance is 75cm. (Note: 1C 1106 C) F k Q1 Q2 r2 (9.0 109 N m 2 / C 2 )(2.6 10 6 C )(2.6 10 6 C ) 158.08 10 3 N 0.158 N 2 (0.75m)