Powerpoint
advertisement

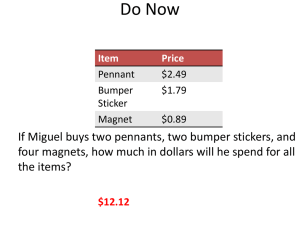
Significant Figures Significant Figures • Engineers often are doing calculations with numbers based on measurements. Depending on the technique used, the precision of the measurements can vary greatly. • It is very important that engineers properly signify the precision of the numbers being used and calculated. Significant figures is the method used for this purpose. Accuracy vs. Precision Accuracy refers to how closely a measured value agrees with the true value Example A scale to increments of 10 lbs is not very precise, but, if it is well calibrated, it is accurate. Courtesy: http://www.chem.tamu.edu/class/fyp/mathrev/mr-sigfg.html Precision vs. Accuracy Precision refers to the level of resolution of the number. Example A scale to increments of tenths of a gram has good precision, however, if it is not well calibrated, it would not be accurate. A scale measures to 0.1 lbs is more precise than one that measures to 1 lbs. Courtesy: College Physics by A. Giambattista, B. Richardson and R. Richardson Significant Figures and Precision In engineering and science, a number representing a measurement must indicate the precision to which the measured value is known. The precision of a device is limited by the finest division on the scale. Example A meterstick, with millimeter divisions as the smallest divisions, can measure a length to a precise number of millimeters and estimate a fraction of a millimeter between two divisions. Courtesy: College Physics by A. Giambattista, B. Richardson and R. Richardson Significant Figures The precision of a quantity is specified by the correct number of significant figures. Significant figures - All the digits that are measured or known accurately + the one estimated digit Example 𝒅 = 𝟏𝟐 𝒌𝒎 d is to the nearest kilometer 2 significant figures 𝒅 = 𝟏𝟐. 𝟎 𝒌𝒎 d is to the nearest tenth of a kilometer 3 significant figures More significant figures mean greater precision!!! Courtesy: College Physics by A. Giambattista, B. Richardson and R. Richardson Rules for Identifying Significant Figures Rule Example Significant digits # Significant Figures 58 5 and 8 2 Final or ending zeroes written to the right of the decimal point are significant. 58.00 5, 8, and zeroes 4 Zeroes written on either side of the decimal point for the purpose of spacing the decimal point are not significant. 0.058 5 and 8 (zeroes are insignificant) 2 Zeroes written between significant figures are significant. 30.058 3, 5, 8 and zeroes 5 Nonzero digits are always significant. Exact Numbers Exact numbers: Numbers known with complete certainty. Exact numbers are often found as conversion factors or as counts of objects. Exact numbers have an infinite number of significant figures. Example Conversion factors : 1 foot = 12 inches Counts of objects: 23 students in a class Courtesy: http://www.chem.sc.edu/faculty/morgan/resources/sigfigs/sigfigs4.html Addition and Subtraction of Significant Figures When quantities are added or subtracted, the number of decimal places (not significant figures) in the answer should be the same as the least number of decimal places in any of the numbers being added or subtracted. Example 50.67 J 0.1 J + 0.9378 J 51.7078 J (2 decimal places - 4 significant fig.) (1 decimal place - 1 significant fig.) (4 decimal places - 4 significant fig.) (4 decimal places - 6 significant fig.) Result: 51.7 J ROUNDING !!! (1 decimal place - 3 sig. fig.) Courtesy: http://www.physics.uoguelph.ca/tutorials/sig_fig/SIG_dig.htm Multiplication, Division, etc., of Significant Figures In a calculation involving multiplication, division, trigonometric functions, etc., the number of significant digits in the answer should be equal to the least number of significant digits in any one of the numbers being multiplied, divided etc. Example 0.097 m-1 (3 decimal places - 2 significant fig.) X 4.73 m (2 decimal places - 3 significant fig. ) 0.45881 (5 decimal places - 5 significant fig.) Result: 0.46 ROUNDING !!! (2 decimal place - 2 sig. fig.) Courtesy: http://www.physics.uoguelph.ca/tutorials/sig_fig/SIG_dig.htm Combination of Operations In a long calculation involving combination of operations, carry as many digits as possible through the entire set of calculations and then round the final result appropriately. DO NOT ROUND THE INTERMEDIATE RESULTS. Example (5.01 / 1.235) + 3.000 + (6.35 / 4.0)= 4.05668... + 3.000 + 1.5875=8.64418... The first division should result in 3 significant figures. The last division should result in 2 significant figures. In addition of three numbers, the answer should result in 1 decimal place. Result: 8.6 ROUNDING !!! (1 decimal place - 2 sig. fig.) Combination of Operations IF YOU ROUND THE INTERMEDIATE RESULTS: Example (5.01 / 1.235) + 3.000 + (6.35 / 4.0)= 4.06 + 3.000 + 1.6=8.66 If first and last division are rounded individually before obtaining the final answer, the result becomes 8.7 which is incorrect. Courtesy:http://www.chem.sc.edu/faculty/morgan/resources/sigfigs/sigfigs4.html Sample Problems PLEASE CHECK PRACTISE: THE FOLLOWING WEBSITES TO • http://www.chem.sc.edu/faculty/morgan/resources/sigfigs /sigfigs8.html • http://science.widener.edu/svb/tutorial/sigfigures.html • http://www.lon-capa.org/~mmp/applist/sigfig/sig.htm