lec1_print

1
Lecture 1
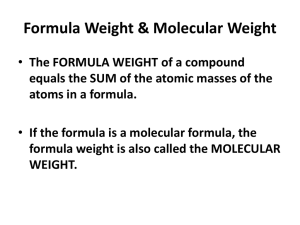
Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place.
2
Today’s lecture
Introduction
Definitions
General Mole Balance Equation
Batch (BR)
Continuously Stirred Tank Reactor (CSTR)
Plug Flow Reactor (PFR)
Packed Bed Reactor (PBR)
3
Chemical Reaction Engineering
Chemical reaction engineering is at the heart of virtually every chemical process. It separates the chemical engineer from other engineers.
Industries that Draw Heavily on Chemical Reaction
Engineering (CRE) are:
CPI (Chemical Process Industries)
Examples like Dow, DuPont, Amoco, Chevron
4
Smog (Ch. 1)
Hippo Digestion (Ch. 2)
Wetlands (Ch. 7 DVD-ROM)
Oil Recovery
(Ch. 7)
Cobra Bites
(Ch. 6 DVD-ROM)
5
Chemical Plant for Ethylene Glycol (Ch. 5)
Lubricant Design
(Ch. 9)
Plant Safety
(Ch. 11,12,13)
6
Materials on the Web and CD-ROM http://www.umich.edu/~essen/
7
Let’s Begin CRE
Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place.
8
Chemical Identity
A chemical species is said to have reacted when it has lost its chemical identity.
The identity of a chemical species is determined by the kind,
number, and configuration of that species’ atoms.
9
Chemical Identity
A chemical species is said to have reacted when it has lost its chemical identity. There are three ways for a species to loose its identity:
1. Decomposition
2. Combination
3. Isomerization
CH
3
CH
3
N
2
H
2
+ O
2
+ H
2
C=CH
2
2 NO
C
2
H
5
CH=CH
2
CH
2
=C(CH
3
)
2
10
Reaction Rate
The reaction rate is the rate at which a species looses its chemical identity per unit volume.
The rate of a reaction (mol/dm 3 /s) can be expressed as either:
The rate of Disappearance of reactant: -r
A or as
The rate of Formation (Generation) of product: r
P
11
Reaction Rate
Consider the isomerization r
A
A B
= the rate of formation of species A per unit volume
-r
A
= the rate of a disappearance of species A per unit volume r
B
= the rate of formation of species B per unit volume
12
Reaction Rate
EXAMPLE: A B
If Species B is being formed at a rate of
0.2 moles per decimeter cubed per second, ie, r
B
= 0.2 mole/dm 3 /s
Then A is disappearing at the same rate:
-r
A
= 0.2 mole/dm 3 /s
The rate of formation (generation of A) is r
A
= -0.2 mole/dm 3 /s
13
Reaction Rate
For a catalytic reaction, we refer to -r
A
', which is the rate of disappearance of species A on a per mass of catalyst basis.
(mol/gcat/s)
NOTE: dC
A
/dt is not the rate of reaction
14
Reaction Rate
Consider species j:
1.
r j is the rate of formation of species j per unit volume
[e.g. mol/dm 3 s]
2.
3.
r j is a function of concentration, temperature, pressure, and the type of catalyst (if any) r j is independent of the type of reaction system (batch, plug flow, etc.)
4.
r j is an algebraic equation, not a differential equation
(e.g. = -r
A
= kC
A or -r
A
= kC
A
2 )
General Mole Balance
F j0
G j
System
Volume, V
F j
15
Molar
Rate of
Species
Flow
j in
Molar
Rate of
Species
Flow j out
Molar
Generation of
Rate
Species j
Molar of
Rate
Accumulati on
Species j
F j 0
F j
G j
dN dt j mole time
mole time
mole time
mole time
16
General Mole Balance
If spatially uniform
G j
r j
V
If NOT spatially uniform
V
1 r j 1
G j 1
r j 1
V
1
V
2 r j 2
G j 2
r j 2
V
2
General Mole Balance
G j
W
r i 1 ji
V i
Take limit
G j
n
i 1 r ji
V i lim V 0 n
r j dV
17
General Mole Balance
F
A0
G
A
System
Volume, V
F
A
18
General Mole Balance on System VolumeV
In Out Generation Accumulation
F
A 0
F
A
r
A dV dN
A dt
Batch Reactor Mole Balance
Batch
19
Well Mixed
F
A 0
F
A 0
F
A
F
A
r
A dV dN
A dt
0
r
A dV dN
A dt
r
A
V
r
A
V
Batch Reactor Mole Balance
Integrating dt dN
A r
A
V when t = 0 N
A t = t N
A
=N
A0
=N
A
t
N
N
A
A 0
dN
A r
A
V
20
Time necessary to reduce number of moles of A from N
A0 to N
A
.
21
Batch Reactor Mole Balance t
N
N
A
A 0
dN
A r
A
V
N
A t
CSTR Mole Balance
CSTR
22
Steady State
F
A 0
F
A
r
A dV dN
A dt dN
A dt
0
CSTR Mole Balance
Well Mixed
r
A dV
r
A
V
F
A 0
F
A
r
A
V 0
23
V
F
A 0
F
A
r
A
CSTR volume necessary to reduce the molar flow rate from
F
A0 to F
A
.
24
Plug Flow Reactor
Plug Flow Reactor Mole Balance
V
25
F
A
F
A
V V
V
In at V
Out
at V
F
A
V
F
A
V
Generation in
V
V
V
r
A
V
0
0
Plug Flow Reactor Mole Balance
Rearrange and take limit as Δ V 0 lim
V 0
F
A V V
V
F
A V r
A
26
dF
A dV
r
A
This is the volume necessary to reduce the entering molar flow rate (mol/s) from F
A0
to the exit molar flow rate of F
A
.
27
Alternative Derivation –
Plug Flow Reactor Mole Balance
PFR
Steady State
F
A 0
F
A
r
A dV
dN dt
A dN
A dt
0
F
A 0
F
A
r
A dV
0
28
Alternative Derivation –
Plug Flow Reactor Mole Balance
Differientiate with respect to V
0 dF
A dV
r
A dF
A dV
r
A
The integral form is:
V
F
A dF
A
F
A 0 r
A
This is the volume necessary to reduce the entering molar flow rate (mol/s) from F
A0 to the exit molar flow rate of F
A
.
Packed Bed Reactor Mole Balance
PBR
29
Steady State
F
A
F
A
W
W
r
A
W
dN
A dt dN
A dt
0
lim
W
0
F
A
W
W
W
F
A
W
r
A
30
Packed Bed Reactor Mole Balance
Rearrange: dF
A dW
The integral form to find the catalyst weight is:
W
F
A
F
A
0 dF
A r
A
PBR catalyst weight necessary to reduce the entering molar flow rate F
A0 to molar flow rate F
A
.
Reactor Mole Balance Summary
31
Reactor Differential
Batch
CSTR
PFR dN
A dt
r
A
V dF
A dV
r
Algebraic Integral t
N
N
A
A 0 dN
A r
A
V
N
A
V
F
A 0
F
A
r
A
V
F
A
F
A
0 dF
A dr
A
F
A t
V
32
Fast Forward 10 weeks from now:
Reactors with Heat Effects
EXAMPLE: Production of Propylene Glycol in an Adiabatic
CSTR
Propylene glycol is produced by the hydrolysis of propylene oxide:
CH
2
CH CH
3
H
2
O H CH
2
CH CH
3
O OH OH
v
0
Propylene Glycol
33
What are the exit conversion X and exit temperature T?
Solution
Let the reaction be represented by
A+B C
34
35
36
37
38
39
Evaluate energy balance terms
40
41
42
Analysis
We have applied our CRE algorithm to calculate the
Conversion (X=0.84) and Temperature (T=614 °R) in a 300 gallon CSTR operated adiabatically.
T=535 °R
A+B C
X=0.84
T=614 °R
43
KEEPING UP
Separations
44
Filtration Distillation
These topics do not build upon one another
Adsorption
Reaction Engineering
45
Mole Balance Rate Laws
These topics build upon one another
Stoichiometry
46
Heat Effects
Isothermal Design
Stoichiometry
Rate Laws
Mole Balance
47
Mole Balance Rate Laws
48
Heat Effects
Isothermal Design
Stoichiometry
Rate Laws
Mole Balance
49
End of Lecture 1
50
Supplemental Slides
Additional Applications of CRE
51
Additional Applications of CRE
52
53
Compartments for perfusion
Alcohol Stomach
V
G
= 2.4 l
Gastrointestinal t
V
G
G
= 2.4 l
= 2.67 min
Liver
V
L t
L
= 2.4 l
= 2.4 min
Central
V
C t
C
= 15.3 l
= 0.9 min Perfusion interactions between compartments are shown by arrows.
V
G
, V
L
, V
C
, and V
M are -tissue water volumes for the gastrointestinal, liver, central and muscle compartments, respectively.
V
54
S is the stomach contents volume.
Muscle & Fat
V
M t
M
= 22.0 l
= 27 min
55
56