Gestational Trophoblastic Disease
advertisement

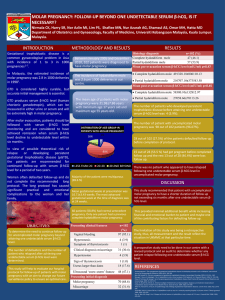
Gestational Trophoblastic Disease Current management Background Incidence – U.S. and Europe 1/1500 South East Asia 1/150 (↓ Carotene, animal fat and Vit. A) Can follow any gestational event – abortion, miscarriage, ectopic, normal pregnancy Curable in vast majority – chemotherapy 1956 Complete & partial mole Invasive mole Placental-site trophoblastic tumour Choriocarcinoma ( always if follows term pregnancy 1/50,000 ) Latter 3 usually derive from molar pregnancy Complete Mole Pathology: Generalized hydatidiform swelling and trophoblastic hyperplasia fetal/embryonic tissue absent Karyotype: 90% 46XX haploid sperm fertilizes ovum and duplicates ovum nucleus either absent or inactivated 10% 46XY Clinical: Diagnosis: Vaginal bleeding 95% Enlarged uterus 50% Theca lutein cysts 50% Hyperemesis 25% PET 25% Hyperthyroidism 5% Trophoblastic emboli 2% (prune juice, anaemia) (often >6cm.- may take 3 months) (no reported case of eclampsia) (if free T4↑ - B-blockade) U/S usually very sensitive – generalized swelling (snow-storm ) Complete Mole Complete Mole Histology Partial Mole Triploid karyotype – extra haploid set paternal Sometimes fetus present – usually triploid Pathology differs from complete: Clinical: growth restricted / multiple anomalies focal hydatidiform swelling varying size of chorionic villi marked villous scalloping focal trophoblastic hyperplasia identifiable embryonic or fetal tissues Usually as a regular incomplete or missed abortion Excessive uterine enlargement / PET very rare No hyperemesis / hyperthyroidism / theca-lutein cysts Diagnosis: U/S may detect focal cystic spaces of varying diameter Diagnosis on histology of curettings Partial Mole Histology Management Pre-operative assessment – medical complications / CXR Evacuation - oxytocin infusion after curettage heavy bleeding should not deter from cervical dilatation suction curettage (fundal massage) uterus usually dramatically reduces in size / bleeding controlled complete with sharp curettage Histological evaluation of all tissue Natural history: Complete - 15% local uterine invasion 4% metastatic disease High risk - hCG > 100000 (40%) large uterus 30% local invasion theca lutein cysts > 6cm. 9% metastases Partial mole - 4% local uterine persistence/no cases choriocarcinoma Many centres have abandoned follow-up Follow up Weekly -hCG (syncytiotrophoblast) levels until normal for 3 consecutive weeks Can take 12-14 weeks Then monthly until normal for 6 months Contraception: Immediate Oral / barrier / permanent No IUCD until hCG normal (perforation) No persistent disease on OCP and regression time not influenced GTN Follow-up WHO Prognostic Scoring Original assessment and scoring system 1984 changed in 2000 Metastatic disease occurs in 4% patients with molar pregnancy Plateau: 4 values over 3 weeks Rise: 10% for 3 values over 2 weeks Clinical examination – especially pelvis, vagina and vulva U/S to exclude pregnancy Brain – MRI superior to CT scan Chest – CXR adequate for counting metastases / CT scan also acceptable Abdomen – CT scan WHO Prognostic Scoring Prognostic factor Age Antecedent pregnancy Interval (months) Pre-treatment -hCG (log) Largest tumour Site of metastases Number of metastases Previous Chemo. Failed Changes: 0 <39 Mole 4 <3 Prognostic score 1 >39 Abortion 4-6 <4 3-4 Spleen Kidney 1-4 2 4 Term pregnancy 7-12 <5 >5 5 GI tract Brain Liver 5-8 >8 Single drug 2 or more ABO group deleted, Liver mets score upgraded, no medium risk group Low risk = 6 High risk = 7 single agent chemotherapy combination chemotherapy FIGO Staging Stage 1 Tumour confined to uterus Stage 2 Tumour confined to pelvis Stage 3 Metastases to lung ( with/without pelvic metastases ) Stage 4 Distant organ metastases ( with/without lung metastases ) Chemotherapy Low-risk If non-metastatic - always curable ( Hysterectomy if chemo fails) Methotrexate: Many regimes I.M. Methotrexate 1mg/Kg days 1,3,5,7 I.M./ P.O. Folinic acid 0.1mg/Kg days 2,4,6,8 I.M. Methotrexate 40mg/m² weekly Actinomycin D: I.V. push 1.25mg/m² every 14 days Follow-up: -hCG, FBC, LFTs and U/Es, creatinine prior to each cycle Continue treatment cycle for 1-3 weeks after normal -hCG Check -hCG monthly for 12 months, then 2 monthly for 12 months Contraception for 12 months Complete remission in 85-90% 80% require only one course Toxicity: Thrombocytopenia 2%, neutropenia 6% and hepatotoxicity 14% High Risk GTN Invasive mole: invades myometrium / diagnosed at hysterectomy / can metastasize mets may be choriocarcinoma Placental site trophoblastic tumour: Locally invasive composed of cytotrophopblast small if any rise in hCG (<3000) vaginal bleeding usually after amenorrhoea Large polypoid tumour / insensitive to chemotherapy Curettage sometimes successful / Hysterectomy Choriocarcinoma: Accounts for majority of metastatic disease Early vascular invasion and widespread dissemination Fragile vessels haemorrhagic complications 80% have lung mets – any respiratory symptom 30% have vaginal mets – highly vascular (avoid biopsy) 10% have liver mets – usually only with extensive tumour elsewhere 10% have brain mets – never isolated ( lung / vagina) Treatment: Prognosis: EMA-CO chemotherapy +/- surgical resection / radiotherapy 75% complete response rate Salvage chemo – BEP varying success Pregnancy After GTN No evidence of increased congenital anomalies after one year contraception Recent Japanese data – Women who concieved during follow-up period < 1 year No adverse effect on anomalies nor preterm delivery Risk of further molar pregnancy: 0.5-2.5% if one previous molar 33% if two previous molar 3 molar pregnancies – poor live birth rate Risk of molar pregnancy increases with number of previous spontaneous abortions Previous term pregnancies reduce risk of GTN Conclusions GTN is rare Ultrasound diagnosis becoming more common Senior staff should perform ERPC ( suction and sharp curettage) Follow-up – clinical and serum -hCG measurements in specialized clinics Chemotherapy curative in vast majority low risk patients