Chapter_2_A_Mathemat..
advertisement

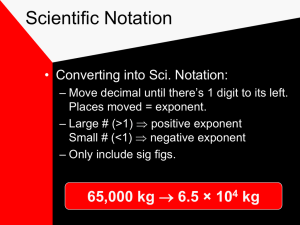
Chapter 2: A Mathematical Toolkit Dr. Zalesinsky 2.2 Measurements with Uncertainties Sig Figs 2.1 Scientific Measurement SI Units Conversions Scientific Notation Operations with Sig Figs 2.3 Visualizing Data • Graphs • Relationships The Metric System • Length or distance is measured in meters • Mass is measured in grams • Volume or capacity is measured in liters • Time is measured in seconds • Energy is measured in Joules • Temperature is measured in Kelvin (not degrees) • Quantity is measured in moles The Liter (L) The meter is slightly longer than a yard The Meter (m) 1 inch = 2.54 cm (this is the only English to metric conversion you must know Gram (g) A gram’s mass is approximately the mass of 2 paperclips. Many medicines are massed by their active ingredients in grams or parts of a gram. The really small and really large SCIENTIFIC NOTATION Scientific Notation Scientific notation is the idea of writing a very large or small number as a power of 10. Move the decimal so that there’s only ONE non-zero digit in front of the decimal and the number of places it has been moved is the exponent. Examples to follow Scientific Notation Examples • Change to Scientific Notation: 985,000,000 0.0000674 9.85 x 108 6.74 x 10-5 Change to Standard Notation: 8.27 x 106 9.565x10-2 8,270,000 .09565 Using Scientific Notation with Calculator Find the EE or EXP button on your calculator (not the 10x nor the SCI buttons) TI Graphing Calculator Examples • Do this calculation: (don’t type in the “x 10 part—use the EE or EXP button) Multiply:(8.76 x 10-10 )(7.9 x1011) = 692 or 6.92x102 Divide: (9.43x1043)/(7.33x1023) = (approximate) 1.3 x1020 be careful to turn your display into correct scientific notation 1.320 is not the same! Uncertainty in Measurement SIGNIFICANT FIGURES English to Metric Comparisons • 1. 2. 3. 4. 5. 6. 7. Which is larger? Meter or yard Mile or Kilometer Gallon or liter Pound or gram Quart or liter Centimeter or inch Gram or ounce • 1. 2. 3. 4. 5. 6. What is the abbreviation for each unit? Meter = _____ Gram = _____ Liter = ______ Second = ______ Joule = _______ Mole = _______ Metric Abbreviations • The BASE units for the metric system are gram, liter, meter, second, Kelvin, Joule and mole. • There are larger and smaller portions of each of these • Their abbreviations come in front of the unit’s abbreviation (ex. centimeter = cm) Larger and Smaller Prefixes • Larger • 1 billion = GIGA (G) • 1 million = MEGA (M) • 1,000 = kilo (k) • 100 = hecto (h) • 10 = deca (D, dk, or da) • • • • • • • Smaller 1trillion = pico (p) 1 billion = nano (n) 1 million = micro (m) 1,000 = milli (m) 100 = centi (c ) 10 = deci (d) Match the abbreviation with the name 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Cm = ________ mg = ________ ML = ________ Gg = ________ ms = ________ km = _______ mL = _______ kJ = ________ mm= _______ Dg = _______ 1. Centimeter 2. Milligram 3. Megaliter 4. Gigagram 5. Microsecond 6. Kilometer 7. Milliliter 8. Kilojoule 9. Millimeter 10. Decagram Conversions Kilo Hecto Deca UNIT 1.0 Deci Centi Milli 100 3.09 2594 73.60 0.000765 0.763 730638 84300 Conversions G M k h D unit d c m m 1.0 3.9 1x10-9 7x 106 403 7.62 27 848 626 n p Significant Figures • Read the correct number of significant figures Measure the following using significant figures Use Sig. Figs to find this measurement Use the correct number of sig. figs in this measurement What digit would be estimated in using Ruler A? A. B. C. D. E. Ones Tenths Hundredths Thousandths Tens What decimal place is estimated when using Ruler B? A. Ones B. Tenths C. Hundredths D. Thousandths E. Tens Measure the width of the rectangle using the correct number of sig figs. A. 3. 75 cm B. 3.6 cm C. 2. 6 cm D. 3.60 cm E. 4.25 cm Measure the length of the rectangle using the correct number of sig figs. A. 12.55 cm B. 12. 5 cm C. 12.0 cm D. 13. 5 cm E. 13.50 cm How many sig figs should be in the correct measurement of the length of this rectangle? A. 2 sig figs B. 3 sig figs C. 4 sig figs D. 5 sig figs E. 1 sig fig The width of this rectangle is 0.90 cm. How many significant figures are in this measurement? A. 3 sig figs B. 2 sig figs C. 1 sig fig D. infinite sig figs E. none of the above Data Table 1.1 Dimension Length (cm) Longer side Width (cm) Shorter side W X Y Z Data Table 1.2 Rectangle W X Y Z Measured Length # of sig figs in Length Measured Width Number of Sig Figs in Width Data Table 1.5 Dimension Length (cm) Longer side Width (cm) Shorter side W X Y Z Data Table 1.6 Rectangle W X Y Z Measured Length # of sig figs in Length Measured Width Number of Sig Figs in Width Calculating with Uncertainty CALCULATIONS WITH SIGNIFICANT FIGURES Multiplication and Division with Sig Figs • The least number of sig figs in the input is equal to the number of sig figs in the answer (output). • Remember all conversion factors and counted numbers have INFINITE sig figs! • Example: 8.03 g x 4.0 cm3/g = ? Addition and Subtraction with Sig Figs • The least number of decimal places in the input is the same number of decimal places in the output (answer). • 12.573 m + 3847.9 m - 378 m = ? • 3482.473 (unrounded) • 3482 rounded to the correct number of decimal places Calculations with Sig Figs 1. A rectangle has a width of 5.0 cm and a length of 8.40 cm. What is the area of this rectangle in cm2? ___________ 2. A rectangular prism has the following measurements: length 8.54 cm, width 7.80cm, and height 10.00 cm. What is the volume in cm3? ______________ Data Table 1.2 Rectangle Measured Length (cm) # of Sig Figs in Length Measured Width (cm) # of Sig Figs in Width W 12.2 3 10.1 3 X 13.6 3 0.7 1 Y 3.3 2 2.4 2 Z 20.2 3 2.5 2 Data Table 1.3 Rectangle Justified # of Sig Fig in Area Unrounded Area Rounded Area (cm2) (cm2) Data Table 1.6 Rectangle Measured Length (cm) # of Sig Figs in Length Measured Width (cm) # of Sig Figs in Width W 12.19 4 10.09 4 X 13.51 4 .61 2 Y 3.27 3 2.49 3 Z 20.19 4 2.38 3 Data Table 1.7 Rectangle Justified # of Sig Fig in Area Unrounded Area Rounded Area (cm2) (cm2) 2.3 VISUALIZING DATA Linear Relationships • Dependent (y) and independent (x) variables when graphed form a straight line • Slope is positive if they are DIRECTLY PROPORTIONAL • Slope is negative if they are INVERSELY PROPORTIONAL 4.5 4 3.5 3 Proportio nal Inverse 2.5 2 No change 1.5 1 0.5 0 1 2 3 4 Nonlinear Relationships • Quadratic equations relate parabolic relationships • Some inverse relationships are hyperbolic Practice Problem • Create a graph from the data chart given • Describe the relationship (linear or non) • If linear, find slope (in/direct) Volume of oil (cc) Concentration (ppm) 0 0 30 1.3 40 1.5 48 2.0 49 3.0 51 11.0