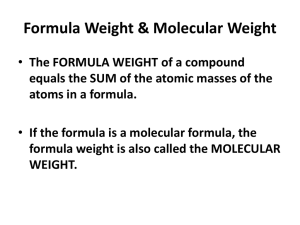
Chapter 2 - Gordon State College
advertisement

Dalton’s Atomic Theory • Elements - made up of atoms • Same elements, same atoms. • Different elements, different atoms. • Chemical reactions involve bonding of atoms The Atom • Made up of: –Protons – (+) charged –Electrons – (-) charged –neutrons Periodic Table • • • • • Alkaline Metals – Grps. I & II Transition Metals Non-metals Halogens – Group VII Noble Gases –Group VIII - little chemical activity Periodic Table • Atomic Mass - # at bottom • how much element weighs • Atomic Number - # on top • gives # protons = # electrons Periodic Table • Atomic Mass –number below the element –not whole numbers because the masses are averages of the masses of the different isotopes of the elements Ions • Are charged species • Result when elements gain electrons or lose electrons 2 Types of Ions • Anions – (-) charged • Example: F- • Cations – (+) charged • Example: Na+ Highly Important! • Gain of electrons makes element (-) = anion • Loss of electrons makes element (+) = cation Isotopes • Are atoms of a given element that differ in the number of neutrons and consequently in atomic mass. Example Isotopes 12C 13C 14C 11C % Abundance 98.89 % 1.11 % –For example, the mass of C = 12.01 a.m.u is the average of 12 13 14 the masses of C, C and C. Determination of Aver. Mass • Ave. Mass = [(% Abund./100) (atomic mass)] + [(% Abund./100) (atomic mass)] Take Note: • If there are more than 2 isotopes, then formula has to be re-adjusted Sample Problem 1 • Assume that element Uus is synthesized and that it has the following stable isotopes: – 284Uus (283.4 a.m.u.) 34.6 % – 285Uus (284.7 a.m.u.) 21.2 % – 288Uus (287.8 a.m.u.) 44.20 % Solution • Ave. Mass of Uus = • [284Uus] (283.4 a.m.u.)(0.346) • [285Uus] +(284.7 a.m.u.)(0.212) • [288Uus] +(287.8 a.m.u.)(0.4420) • = 97.92 + 60.36 + 127.21 • = 285.49 a.m.u (FINAL ANS.) Oxidation Numbers • Is the charge of the ions (elements in their ion form) • Is a form of electron accounting • Compounds have total charge of zero (positive charge equals negative charge) Oxidation States • Are the partial charges of the ions. Some ions have more than one oxidation states. Oxidation States • - generally depend upon the how the element follows the octet rule • Octet Rule – rule allowing elements to follow the noble gas configuration Nomenclature • - naming of compounds Periodic Table • Rows (Left to Right) - periods • Columns (top to bottom) groups Determination of Aver. Mass • Ave. Mass = [(% Abund./100) (atomic mass)] + [(% Abund./100) (atomic mass)] Sample Problem 1 • Assume that element Uus is synthesized and that it has the following stable isotopes: –284Uus (283.4 a.m.u.) 34.6 % –285Uus (284.7 a.m.u.) 21.2 % 288 – Uus (287.8 a.m.u.) 44.20 % Solution • Ave. Mass of Uus = • [284Uus] (283.4 a.m.u.)(0.346) • [285Uus] +(284.7 a.m.u.)(0.212) • [288Uus] +(287.8 a.m.u.)(0.4420) • = 97.92 + 60.36 + 127.21 • = 285.49 a.m.u (FINAL ANS.) STOICHIOMETRY –For example, the mass of C = 12.01 a.m.u is the average of 12 13 14 the masses of C, C and C. Formula Weight & Molecular Weight • The FORMULA WEIGHT of a compound equals the SUM of the atomic masses of the atoms in a formula. • If the formula is a molecular formula, the formula weight is also called the MOLECULAR WEIGHT. The MOLE • Amount of substance that contains an Avogadro’s number (6.02 x 10 23)of formula units. The MOLE • The mass of 1 mole of atoms, molecules or ions = the formula weight of that element or compound in grams. Ex. Mass of 1 mole of water is 18 grams so molar mass of water is 18 grams/mole. Formula for Mole Mole = mass of element formula weight of element Sample Mole Calculations 1 mole of C = 12.011 grams » 12.011 gm/mol • 0.5 mole of C = 6.055 grams » 12.011 gm/mol Avogadro’s Number • Way of counting atoms • Avogadro’s number 23 = 6.02 x 10 Point to Remember One mole of anything is 6.02 23 x 10 units of that substance. And…….. • 1 mole of C has the same number of atoms as one mole of any element Formula Weight & Molecular Weight • The FORMULA WEIGHT of a compound equals the SUM of the atomic masses of the atoms in a formula. • If the formula is a molecular formula, the formula weight is also called the MOLECULAR WEIGHT. Summary • Avogadro’s Number gives the number of particles or atoms in a given number of moles • 1 mole of anything = 6.02 x 10 atoms or particles 23 Sample Problem 2 • Compute the number of atoms and moles of atoms in a 10.0 gram sample of aluminum. Solution • PART I: • Formula for Mole: –Mole = mass of element atomic mass of element Solution (cont.) • Part II: atoms To determine # of • # atoms = moles x Avogadro’s number Problem # 2 • A diamond contains 5.0 x 1021 atoms of carbon. How many moles of carbon and how many grams of carbon are in this diamond? Molar Mass • Often referred to as molecular mass –Unit = gm/mole • Definition: –mass in grams of 1 mole of the compound Example Problem • Determine the Molar Mass of C6H12O6 Solution • Mass of 6 mole C = 6 x 12.01 = 72.06 g • Mass of 12 mole H = 12 x 1.008 = 12.096 g • Mass of 6 mole O = 6 x 16 = 96 g • Mass of 1 mole C6H12O6 180.156 g = Problem #3 • What is the molar mass of (NH4)3(PO4)? Molar Mass • Often referred to as molecular mass –Unit = gm/mole • Definition: –mass in grams of 1 mole of the compound Sample Problem • • • • • • • • • • Given 75.99 grams of (NH4)3(PO4), determine the ff: 1. Molar mass of the compound 2. # of moles of the compound 3. # of molecules of the compound 4. # of moles of N 5. # of moles of H 6. # of moles of O 7. # of atoms of N 8. # of atoms of H 9. # of atoms of O