Chemical Quantities Lecture
advertisement

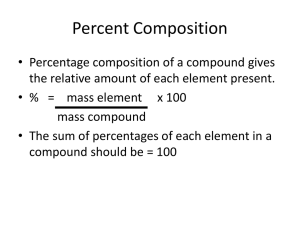
CHEMICAL QUANTITIES THE MOLE CONCEPT AND ITS APPLICATIONS CHEMICAL QUANTITIES OBJECTIVES 1. STUDENTS WILL BE ABLE TO UNDERSTAND AND KNOW THE DEFINITIONS OF THE MOLE AND AVOGADRO’S NUMBER RELATIONSHIP BETWEEN MOLES AND MASS RELATIONSHIP BETWEEN MOLES AND PARTICLES CALCULATE THE FORMULA MASS OF A COMPOUND GIVEN THE PERIODIC TABLE CALCULATE THE MASS PERCENT COMPOSITION OF A COMPOUND GIVEN THE FORMULA, FORMULA MASS, AND PERIODIC TABLE. DRILL & OBJECTIVES How many eggs are there in a dozen? 1. If I bought 3 dozen eggs, how many eggs do I have? 1. What are some ways that you can measure things? (such as the amount of a substance) 2. OBJECTIVES 1. STUDENTS WILL BE ABLE TO UNDERSTAND AND KNOW THE DEFINITIONS OF THE MOLE AND AVOGADRO’S NUMBER RELATIONSHIP BETWEEN MOLES AND MASS RELATIONSHIP BETWEEN MOLES AND PARTICLES CALCULATE THE FORMULA MASS OF A COMPOUND GIVEN THE PERIODIC TABLE CALCULATE THE MASS PERCENT COMPOSITION OF A COMPOUND GIVEN THE FORMULA, FORMULA MASS, AND PERIODIC TABLE. CHEMICAL QUANTITIES How many eggs are there in a dozen? 12! CHEMICAL QUANTITIES How many roses are there in a dozen roses? 12! CHEMICAL QUANTITIES Does it matter what the _____________ is? CHEMICAL QUANTITIES Does it matter what the _____________ is? NO! A Dozen is a dozen! No matter if it’s flowers, eggs, bagels, ect. It represents a number of _______ CHEMICAL QUANTITIES In Chemistry the same concept is valid, Let’s talk about “The Mole” and how it relates to Chemistry… THE MOLE? The Mole? THE MOLE? “The Mole” or “A Mole” is an amount of a substance There are 6.02 x1023 atoms (representative particles of a substance) in 1 Mole (of that substance). This number (6.02 x1023) is called Avogadro’s number Named after Amedeo Avogadro di Quarenga (1776 – 1856), an Italian scientist. THE MOLE! Let’s put this into perspective How much is 1 mol of a substance? 6.023 x 23 10 !! THE MOLE! IF you had 6.02 X 1023 Watermelon Seeds… THE MOLE! IF you had 6.02 X 1023 Watermelon Seeds… it would be found inside a watermelon slightly larger than the moon! THE MOLE! If you had 6.02 X 1023 Grains of Sand… THE MOLE! IF you had 6.02 X 1023 Grains of Sand… it would be more than ALL the sand on Miami Beach. THE MOLE? A mole is the amount of substance or representative particles of any substance. Therefore, as we have just seen 1 mol of N2 there are 6.02x1023 MOLECULES of N2 1 mol of C12H22O11 has how many molecules? 6.02x1023 can be used for “atoms” if they are the same element OR It can be used for “molecules” for many elements that are together, via compounds. CHEMICAL QUANTITIES How many moles of Magnesium is 3.01x1022 atoms of magnesium? Step 1 - What do we know? We know that there are 3.01x1022 atoms of Mg Step 2 - What do we want know? We want to know the number of moles of Mg Step 3 – What do we know that can be used for these conversions? We know that there are 6.02x1023 atoms in 1 mol Step 4 – Solve the problem CHEMICAL QUANTITIES How many moles of Magnesium is 3.01x1022 atoms of magnesium? 3.01x1022 atoms Mg X 1 mol Mg 6.02x1023 atoms of Mg = 5.00x10-2 mol Mg CHEMICAL QUANTITIES Individual Problems How many moles are 1.20x1025 atoms of phosphorus? How many atoms are in 0.750 mol of Zinc? How many molecules are there in 4 mol of glucose, C6H12O6? How many molecules are there in 0.44 mol N2O5 CHEMICAL QUANTITIES Now there is a difference between asking how many atoms are there in the entire compound and how many atoms of one of the compound there are. We said that there are the same number or atoms in a compound or a diatomic molecule. However, there is a difference between these questions… How many atoms are there in aluminum fluoride? VS. How many fluoride ions are there in aluminum fluoride? CHEMICAL QUANTITIES So let’s answer the question. How many fluoride ions are there in 1.46 mol of aluminum fluoride? If we follow the four steps that we went through prior CHEMICAL QUANTITIES How many fluoride ions are there in 1.46 mol of aluminum fluoride? Step 1 - What do we know? We know that there are 1.46 mol of aluminum fluoride Step 2 - What do we want know? We want to know the number of F- Step 3 – What do we know that can be used for these conversions? NO! We know that there are 6.02x1023 atoms in 1 mol BUT is that enough information?? CHEMICAL QUANTITIES How many fluoride ions are there in 1.46 mol of aluminum fluoride? We want to know the number of FBut how can we find out the number of fluoride ions we have from what is given? We can get the information we need by writing out the chemical formula of the compound. AlF3 CHEMICAL QUANTITIES How many fluoride ions are there in 1.46 mol of aluminum fluoride? 1.46 mol AlF3 X 6.02x1023 Formula units of AlF3 3 F- ions 1 formula unit AlF3 1 mol AlF3 X = 26.3676 x 1023 F- ions INDIVIDUAL WORK How many ammonium ions are in 0.036 mol ammonium phosphate, (NH4)3PO4? DRILL What is Avogadro's number? How many particles are there in 1 mol of a substance? How many atoms are there in 1.45 mol of Na? CHEMICAL QUANTITIES THE GRAM FORMULA MASS CHEMICAL QUANTITIES Chemists have defined the gram atomic mass as the number of grams of an element that is numerically equal to the atomic mass in amu The Gram atomic mass is the mass of one mole of atoms of a monatomic element This can also be described as MOLAR MASS – in place of gram formula mass to refer to the mass of a mole of any element or compound. I will be using the term Molar Mass more frequently CHEMICAL QUANTITIES Atomic Mass Number CHEMICAL QUANTITIES This number tells you how many grams of this element there are in 1 mol of the element Therefore, there are 47.88 grams/mol of Ti Or there are 47.88 grams of Ti IN 1 mol of Ti CHEMICAL QUANTITIES B C N O F 10.811g 12.0107g 14.0067g 15.9994g 18.998g The molar mass in grams/mol CHEMICAL QUANTITIES You can calculate the total molecular weight of a molecule by adding up the molar masses of each element. The molar mass of the molecule SO4 is equal to S = 32.065 g/mol x 1 S = 32.065 g/mol O = 15.9994 g/mol x 4 O = 63.9976 g/mol SO4 = 96.0626 g/mol I will specify the amount of significant figures needed in your answer INDIVIDUAL WORK What is the molar mass of the following compounds? REMEMBER YOUR UNITS! 1. 2. 3. 4. 5. PCl3 Sodium Carbonate C8H18 Aluminum Sulfate (NH4)2CO3 CHEMICAL QUANTITIES Why is this important? This is important because it will allow us to covert from moles to grams and grams to moles, allowing for quantitative experimentation. Let’s do a calculation… How many grams are in 7.20 mol of dinitrogen trioxide? CHEMICAL QUANTITIES How many grams are in 7.20 mol of dinitrogen trioxide? Step 1 – add up the total molar mass of the compound If it is in word form, write the chemical formula Step 2 – Set up the proper conversion factors Step 3 – Solve CHEMICAL QUANTITIES How many grams are in 7.20 mol of dinitrogen trioxide? Step 1 – add up the total molar mass of the compound If it is in word form, write the chemical formula N2O3 N = 14.0 g/mol x 2 N = 28.0 g/mol O = 16.0 g/mol x 3 O = 48.0 g/mol N2O3 = 76.0 g/mol CHEMICAL QUANTITIES How many grams are in 7.20 mol of dinitrogen trioxide? Step 2 – Set up the proper conversion factors Step 3 – Solve 7.20 molN 2 O 3 x 76 .0 gN 2 O 3 1.00 molN 2 O 3 547 .2 gN 2 O 3 INDIVIDUAL WORK Find the mass of the following 1. 2. 3. 3.32 mol K 5.08 mol Ca(NO3)2 4.52x10-3 mol K2CO3 Find the number moles of the following 1. 2. 3. 0.000264g Li2HPO4 847g (NH4)2CO3 195g calcium nitrate EXIT TICKET Does 54.938 g of Mn have the same number of moles as that of 112.411 g of Cd? Explain why or why not. DRILL What is Avogadro’s number? How many moles are there in 245 kg of CH2COOH? Does 54.938 g of Mn have the same number of moles as that of 112.411 g of Cd? Explain why or why not. Group Work Find the mass (g) of the following: 1. 2. 3. 4. 5. 6. 10.0 mol Cr 2.20x10-3 mol Sn 0.720 mol Be 2.40 mol N2 4.52x10-3 mol C20H42 0.0112 mol Potassium Carbonate Find the number of moles of the following: 1. 1. 2. 3. 4. 5. 72.0 g Ar 3.70x10-1 g B 333 g Tin (II) Fluoride 7.21x10-2 g He 27.4 g TiO2 CHEMICAL QUANTITIES THE VOLUME OF A MOLE OF GAS CHEMICAL QUANTITIES How would we measure the amount of moles in a gas? CHEMICAL QUANTITIES The volume of a gas is usually measured at STP (Standard Temperature and Pressure) STP conditions Temperature 0°C Pressure 1 atmosphere (atm) CHEMICAL QUANTITIES At STP conditions 1 mol of ANY gas occupies a volume of 22.4 Liters (L) 22.4 Liters of a gas / 1 mol of the gas 22.4 L of a gas contains 6.02 x 1023 representative particles of that gas This is known as the molar volume of a gas CHEMICAL QUANTITIES What were the units of the molar volume of a gas? Liters What kind of unit is liters? VOLUME Therefore, 1 mol of any gas occupies the same volume not mass CHEMICAL QUANTITIES He 22.4 L N2 6.02 x 1023 molecules of N2 28g of N2 Ne 22.4 L He 6.02 x 1023 molecules of He 4g 22.4 L CO2 6.02 x 1023 molecules of CO2 44g of CO2 CO2 INDIVIDUAL WORK What is the volume at STP of these gases? 5.40 mol O2 3.20 x 10-2 mol CO2 Assuming STP conditions, how many moles are there in these volumes 74.6 L SO2 5.78x10-2 N2 CHEMICAL QUANTITIES Because the molar volume of a gas is a VOLUME, we can relate density (mass over volume) of a gas to determine the mass of the gas. If we have a gas that has a density of 1.964 g/L we can multiply the density with the molar volume of a gas (22.4 L) to calculate the mass of the gas. 1.964 g x 22.4 L = 44.0g L GROUP WORK The densities of gases A, B, & C are 1.25 g/L, 2.86 g/L, and 0.714 g/L, respectively. Calculate the molar mass of each of these substances and compare them to the molar mass of ammonia, sulfur dioxide, chlorine, nitrogen, and methane Identify the gasses A, B, & C CHEMICAL QUANTITIES Atoms (6.02 x 10 23) 1mol 1 mol Molar Mass 22.4 L Solids and Liquids Gases HOMEWORK PROBLEMS COPY THEM DOWN! 1. Calculate the volume of each of these gases at STP 1. 2. 9.6 mol He 4.8 mol N2 How many moles is each of the following, assuming STP 2. 1. 2. 56.o L N2O 0.224 L O2 Find each of the following quantities 3. 1. 2. The mass of 18.0L of CH4 (STP) The volume in liter, of 835 g of SO3 (STP) DRILL What are STANDARD conditions? How many liters are there in 1 mol of gas? Does it matter which gas it is? Why or why not? CHEMICAL QUANTITIES PERCENT COMPOSITION CHEMICAL QUANTITIES- % COMPOSITION Calculating Percent Composition Percent Composition The percent by mass of each element in a compound They must add up to 100% Ex. K2CrO4 40.3% K 26.8% Cr 32.9% O 100% Total CHEMICAL QUANTITIES - % COMPOSITION Percent Mass of an element in a compound is the number of grams of the element divided by the grams of the compound, multiplied by 100% % mass = Grams of element Grams of compound x 100% CHEMICAL QUANTITIES- % COMPOSITION Let’s look at our example again K2CrO4 STEP 1 Calculate the Total Molecular Mass of K2CrO4 – Go ahead a calculate that now K = 39 g x 2K = 78 g Cr = 52 g O = 16 g x 4O = 64g K2CrO4 = 194g CHEMICAL QUANTITIES- % COMPOSITION Let’s look at our example again K2CrO4 STEP 2 Using the individual molar mass of each element, divide each element with the total molar mass Go ahead and calculate that now with 3 Significant figures K = 39 g x 2K = 78 g K ÷194g = .402 Cr = 52 g ÷194g = 0.268 O = 16 g x 4O = 64g ÷194g = 0.330 CHEMICAL QUANTITIES- % COMPOSITION Let’s look at our example again K2CrO4 STEP 3 Multiply the number calculated from Step 2 and multiply by 100% Go ahead and calculate that now K = 39 g x 2K = 78 g K ÷194g = .402 x 100% = 40.2% K Cr = 52 g ÷194g = 0.268 x 100% = 26.8% Cr O = 16 g x 4O = 64g ÷194g = 0.330 x 100% = 33% O CHEMICAL QUANTITIES- % COMPOSITION INDIVIDUAL WORK Calculate the mass of carbon in 82g in C3H8 Calculate the percent composition of each of these compounds. 1. 2. 1. 2. 3. 4. Propane, C3H8 Sodium bisulfate, NaHSO4 Calcium acetate, Ca(C2H3O2)2 Hydrogen cyanide, HCN CLOSURE Take the remainder of this period and write down in complete sentences what you learned today, approximately 2-3 paragraphs. DRILL What is percent composition? What is the formula for percent composition? DRILL Calculate the mass of oxygen in 142g of Sodium bisulfate, NaHSO4 CHEMICAL QUANTITIES EMPIRICAL FORMULAS CHEMICAL QUANTITIES- EMPIRICAL FORMULAS Empirical Formula Gives the lowest whole number ratio of the elements in a compound. This ratio is not necessarily the molecular formula! Calculating the empirical formula is going to require the use of almost everything you’ve learned so far. Each element in the compound would require different amounts depending on the mass and % composition CHEMICAL QUANTITIES- EMPIRICAL FORMULAS Steps to solve for an Empirical Formula 1. Write down the base formula AxBY 2. Convert whatever you have into moles using dimensional analysis 3. Divide each element by the lowest number of moles in the compound 4. Round if necessary to the nearest 10ths place and/or multiply by a number to get a whole number ratio 5. Write down the Empirical Formula CHEMICAL QUANTITIES- EMPIRICAL FORMULAS What is the empirical formula of a compound that is 25.9% Nitrogen and 74.1% Oxygen? STEP 1 - Write down the base formula AxBY… NxOy CHEMICAL QUANTITIES- EMPIRICAL FORMULAS What is the empirical formula of a compound that is 25.9% Nitrogen and 74.1% Oxygen? STEP 2 - Convert whatever you have into moles using dimensional analysis Because this is a % composition, we know that everything has to add up to 100%; therefore, we can assume that if we had 100g total of the compound, there would be 25.9g of N and 74.1g of O CHEMICAL QUANTITIES- EMPIRICAL FORMULAS What is the empirical formula of a compound that is 25.9% Nitrogen and 74.1% Oxygen? STEP 2 - Convert whatever you have into moles using dimensional analysis 25.9g of N x 1 mol N 14.0 g N = 1.85 mol N 74.1g of O x 1 mol O 16.0 g O = 4.63 mol O CHEMICAL QUANTITIES- EMPIRICAL FORMULAS What is the empirical formula of a compound that is 25.9% Nitrogen and 74.1% Oxygen? STEP 3 - Divide each element by the lowest number of moles in the compound 25.9g of N x 1 mol N 14.0 g N = 1.85 mol N ÷ 1.85 mol = 1 mol N 74.1g of O x 1 mol O 16.0 g O = 4.63 mol O ÷ 1.85 mol = 2.5 mol O CHEMICAL QUANTITIES- EMPIRICAL FORMULAS What is the empirical formula of a compound that is 25.9% Nitrogen and 74.1% Oxygen? STEP 4 - Round if necessary to the nearest 10ths place and/or multiply by a number to get a whole number ratio 2 = 2 mol N 1 mol N x ____ 2 = 5 mol O 2.5 mol O x ____ CHEMICAL QUANTITIES- EMPIRICAL FORMULAS What is the empirical formula of a compound that is 25.9% Nitrogen and 74.1% Oxygen? STEP 5 - Write down the Empirical Formula 2 mol N 5 mol O N2O5 CHEMICAL QUANTITIES- MOLECULAR FORMULAS The Molecular formula is the actual formula of a molecular compound While the empirical formula give us the base ratio of the compound, the molecular formula will give the actual molecular formula by using the molar mass. x (empirical formula) = molecular formula Ratio of the compounds Chemical Quantities- Molecular Formulas Therefore, if you have the molar mass of the compound and divide it by the molar mass of the empirical formula, you will get the ratio between the two. Multiply the ratio to each element of the compound to get the the molecular formula. Chemical Quantities- Molecular Formulas Ex. If the empirical formula was calculated to be CH4N and the known molecular formula molar mass is = to 60g 1st – Take the molar mass of the empirical formula CH4N In this case it is calculated to be 30g/mol 2nd – Divide the known molar mass of 60g/mol and divide it by 30 g/mol 60/30 = 2 3rd – Take the ratio of the two molar masses and then multiply it by each element of the compound C1x2H4x2N1x2= C2H8N2 Chemical Quantities- Molecular Formulas Write the Molecular Formula for the problem below The compound methyl butanoate has a percent composition of 58.8% C, 9.8% H, 31.4%O. If its molecular mass is 102 g/mol, what is it’s molecular formula? Write the Empirical Formula for the following 1. 79.8% C 20.2% H 2. 67.6% Hg 10.8% S 21.6% O 3. 27.59% C 1.15% H 16.09%N 55.17%O 4. 17.6% Na 39.7%Cr 42.7%O DRILL What is the difference between an empirical formula and molecular formula? Chemical Quantities – Review Determine the Empirical Formula with the parameters below 1. 71.72% Cl, 16.16% O, and 12.12%C What is the molecular formula for the compound below? The compound’s empirical formula and molar mass is given Below 2. HgCl, 472.2 g/mol Determine the molecular formula for the compound 3. 94.1%O and 5.9% H ; molar mass = 34g Find the empirical formula for each compound from its percent composition 4. 5. 72.4% Fe and 27.6% O 52.8% Sn, 12.4% Fe, 16.0% C, and 18.8% N