Compounds & Moles

COMPOUNDS & MOLES
Unit 5
Overview
Naming
Ionic
Covalent
Acids
Simple Organic
The Mole
Molar Mass
Mole Conversions
Calculations
Percent Composition
Empirical Formula
Molecular Formula
Why do we name compounds?
Think of some common compounds that you know of
H
2
O = water
CaCO
3
= limestone
NaCl = table salt
Imagine if we had to memorize common names for the millions of known compounds that we had today
…IMPOSSIBLE!
Standard system was created to name compounds
IUPAC (International Union of Pure and Applied
Chemistry)
Chemical Formulas
Indicate the relative numbers of atoms of each kind in a chemical compound
Indicates 8 carbon atoms
C
8
H
18
Indicates 18 hydrogen atoms
Molecular vs. Structural Formulas
Molecular Formula
Lists elements in a compound and how many of each element you have
Example: C
2
H
6
O
Structural Formula
Shows how atoms are “connected” in the structure
Example CH
3
CH
2
OH or CH
3
OCH
3
Monatomic Ions
Ions formed from a single atom
Naming cations
Simply give the element’s name
Example
Ca +2 = calcium ion
Na +1 = sodium ion
Naming anions
Drop the ending of the element’s name and add “-ide”
Example
F -1 = fluoride ion
O -2 = oxide ion
Binary Ionic Compounds
Ionic compound composed of 2 elements
Writing Names
Name the cation 1st
Name the anion 2 nd
Example:
NaCl = sodium chloride
MgF
2
Sr
3
N
2
= magnesium fluoride
= strontium nitride
Binary Ionic Compounds
Writing Formulas
Example: aluminum oxide
Write the symbols for the ions side by side (cation first)
Al +3 O -2
Criss-cross the charges (use absolute value)
Al
2
O
3
Simplify (divide both numbers by largest common factor)
Al
2
O
3
Binary Ionic Compounds
More examples (name to formula)
Calcium nitride = Ca
3
N
2
Potassium sulfide = K
2
S
Magnesium oxide = MgO
Polyatomic Ions
Electrically charged group of two or more atoms
Oxyanion – polyatomic anion that contains oxygen
General naming rules
Most common oxyanion ends in “-ate”
Example
ClO
3
-1
NO
3
-1
SO
4
-2
= chlorate
= nitrate
= sulfate
Polyatomic Ions
The number of oxygen atoms may be altered giving new endings and prefixes to oxyanions
1 more oxygen = per_______ate
Common form = _______ate
1 less oxygen = _______ite
2 less oxygens = hypo_______ite
Example
ClO
4
-1 = perchlorate
ClO
ClO
3
-1
2
-1
= chlorate
= chlorite
ClO -1 = hypochlorite
Notice that the charge of the oxyanion does not change (only the number of oxygen atoms)
Polyatomic Ions
Ionic compounds (contain “ions”)
Writing Name
If ion comes first, name the polyatomic ion then name the anion
If the ion comes second, name the cation then name the polyatomic ion (do not change ending)
Examples
NH
4
Cl = ammonium chloride
CaSO
4
= calcium sulfate
Ba
3
(PO
4
)
2
= barium phosphate
Polyatomic Ions
Writing Formula
Follow same rules as binary ionic compound, but when charges are criss-crossed, use parenthesis to indicate number belongs to entire polyatomic ion
Example: calcium nitrate
Ca
+2
NO
3
-1
=
Ca(NO
3
)
2
Stock System (Ionic Compounds)
For elements that form two or more cations with different charges (example Pb +2 and Pb +4 )
Uses roman numeral to indicate ion’s charge
Transition metals, Sn, and Pb use this system
Writing Formulas
Roman numeral indicates charge of the cation (use that to criss cross)
Examples
Copper (II) bromide = CuBr
2
Iron (III) sulfide = Fe
2
S
3
Tin (IV) phosphate = Sn
3
(PO
4
)
4
Stock System (Ionic Compounds)
Writing Names
Use the anion (known charge) that the cation is bonded to and solve for the charge of the cation
Total positive charge (from cation) must equal total negative charge (from anion)
Example: VF
6
Fluorine has a charge of -1
There are six fluorines bonded to the vanadium
6 × -1 = -6 so the charge of vanadium is 6
Name = vanadium (VI) fluoride
Stock System (Ionic Compounds)
Example 2: Sn
3
N
2
The charge of nitrogen is -3
There are 2 nitrogen atoms
2 × -3 = -6
There are 3 tin atoms that add up to a charge of +6
+6 ÷ 3 = -2 so each tin atom has a charge of +2
Name = tin (II) nitride
Exception: some transition metals only have one charge
(nickel, silver, zinc, etc.) so the roman numeral is omitted
Prefixes
Used in naming covalent compounds
Indicate how many of each atom you have
Number Prefix
1
2 monodi-
5
6
3
4 tritetrapentahexa-
7
8
9 heptaoctanona-
10 deca-
Binary Covalent Compounds
Writing Names
Name the cation followed by the anion (-ide ending)
Use prefixes to indicate how many of each atom you have
Examples:
P
4
Br
10
Si
2
O
5
= tetraphosphorous decabromide
= disilicon pentoxide
Note
If an o or a are doubled, drop the o or a of the prefix
Never use mono- on cation (only on anion)
Binary Covalent Compounds
Writing Formulas
Prefix indicates how many of each atom you have
Do not criss-cross numbers
Examples:
Trinitrogen octachloride = N
3
Cl
8
Arsenic tetrabromide = AsBr
4
Summary
When writing names of formulas…
YES
Is it ionic?
NO
YES
Is the cation a transition metal,
Sn, or Pb?
NO
Use Roman numerals
Name cation then anion (write it like it is)
Use prefixes
(covalent)
Acids
Binary acid – contains two elements (one usually hydrogen and the other usually a halogen)
Oxyacid – acids that contain hydrogen, oxygen, and a third element (usually a nonmetal)
Usually hydrogen and a polyatomic ion
Acids
Naming binary acids
Use form of hydro_____ic acid
Examples:
HF = hydrofluoric acid
HCl = hydrochloric acid
Acids
As the number of oxygen atoms changes in oxyacids, so does the name (just like the oxyanions)
1 more oxygen = per_______ic acid
Common form = _______ic acid
1 less oxygen = _______ous acid
2 less oxygens = hypo_______ous acid
Example
HClO
4
HClO
3
HClO
2
= perchloric acid
= chloric acid
= chlorous acid
HClO = hypochlorous acid
Carbon
• Basis for all life.
• Study of carbon compounds is called organic chemistry.
• Can form single, double and triple bonds.
• Long carbon chains can be produced.
• Will bond with many other elements.
• A HUGE number of compounds is possible (organic compounds)
Naming Simple Organic Compounds
Organic compounds containing only carbon and hydrogen are called hydrocarbons
Alkane – all carbons form single bonds
Alkene – carbons form double bonds
Alkyne – carbons form triple bonds
Whether a compound is an alkane, alkene, or alkyne determines the suffix (ending) in the name of the hydrocarbon
Naming Simple Organic Compounds
Prefix Carbons
Meth1
Eth-
Prop-
2
3
But-
Pent-
Hex-
Number of carbons determines prefix used in name
4
5
6
Hept-
Oct-
Non-
Dec-
7
8
9
10
Naming Simple Organic Compounds
Examples
CH
4
C
2
H
6
= meth ane
= eth ane prop ane prop ene prop yne
The Mole
The amount of a substance that contains as many particles as there are atoms in exactly 12 g of 12 C
SI unit of amount of a substance
Abbreviated “mol”
Counting unit just like a “dozen”
1 dozen donuts is the same amount as 1 dozen books
1 mole of hydrogen atoms is the same amount as 1 mole of sodium atoms
Avogadro’s Number
6.022×10 23 of anything is a mole
Named after Italian scientist Amadeo Avogadro
Experimentally determined number of atoms in 12 grams of 12 C
How big is 602,200,000,000,000,000,000,000?
One mole of donut holes would cover the Earth 5 miles deep in the donut holes
One mole of pennies stacked on top of each other would reach from the
Earth to the moon 7 times
If you started counting when you were born and never stopped until the day you died, you would never come close to reaching 6.022×10 23
Avogadro’s Number
1 Liter of water contains 55.5 moles of H
2
O
A 5 lb bag of sugar contains 6.6 moles of sugar
How can that be?!
Atoms and molecules are so tiny that when we use units of moles (
6.022×10
23 ) it puts the particles into measurable quantities
Molar Mass
1 mole of hydrogen atoms = 1 mole of sodium atoms
BUT…
1 mole of hydrogen atoms DOES NOT have the same mass as 1 mole of sodium atoms
Individual atoms have different masses
They are the same amount but not the same mass
Molar Mass
The periodic table tells us the mass of 1 mole of any atom
It’s the same as the average atomic mass/relative atomic mass (decimal number on the table)
Molar Mass – mass of 1 mole of an atom or compound
Units are “grams/mole” or “g/mol”
Molar Mass
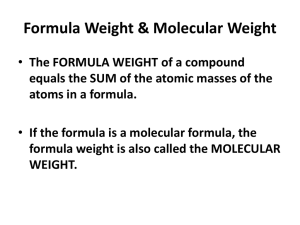
To find the molar mass of a compound, add the molar masses of all atoms in a compound
Also called formula mass or molecular mass (compounds only)
Example: CO
2
(1 atom of C and 2 atoms of O)
1 atom C x 12.011 = 12.011
2 atoms O x 15.9994 = 31.9988
Molar mass = 44.010 g/mol
Mole Relationships
Atoms
Molecules
6.02 x 10 23
Mole
Molar Mass
Grams
To go between units of grams, moles and atoms (or molecules) use conversions!
6.022×10 23 is how many atoms or molecules are in 1 mole of any substance
The molar mass is how many grams are in one mole of any substance
Mole Conversions
How many grams are in 5.0 moles of calcium?
5.0 mole ×
40.078 g
1 mole
= 200.39 g
How many atoms are in 2.1 moles of xenon?
2.1 moles ×
6.022×10 23 atoms
1 mole
= 1.26×10 24 atoms
Mole Conversions
There is no way to go straight from grams to atoms or molecules in one step
Must use moles as the intermediate step
How many atoms are in 9.8 g of Pb?
9.8g ×
1 mol
207.2 g
×
6.022×10 23 atoms
1 mole
= 2.8×10 22 atoms
Mole Conversions
When a conversion includes a compound, it will use the word molecules when a conversion includes an element, it will use the word atoms
There are still as many molecules in a mole as there are atoms
How many grams are in 3.4×10 22 molecules of H
2
O?
First solve for molar mass of H
2
O
(H
2
O molar mass = 18.02g/mol)
3.4×10 22 molecules ×
1 mole
6.022×10 23 molecules
×
18.02 g
1 mole
= 1.0 g
Percent Composition
Percentage by mass of each element in a compound
Example: What is the percent composition of BaSO
4
?
Molar Mass part ÷ total
Multiply by 100
Ba = 1 × 137.3 = 137.3 (137.3/233.4) ×100= 58.8% Ba
S = 1 × 32.1 = 32.1
(32.1/233.4) ×100= 13.8% S
O = 4 × 16.0 = 64.0
(64.0/233.4) ×100= 27.4% O
233.4
Total molar mass
Empirical Formula
Smallest whole-number ratio formula of a compound
Simplest formula
What is the empirical formula of a compound that is 27.0% sodium, 16.5% nitrogen, and 56.5% oxygen by mass?
Assume that you have a 100 gram sample
Divide by
Molar Mass smallest number
Na 27.0/22.99 = 1.17 /1.17 = 1
N 16.5/14.01 = 1.18 /1.17 = 1
O 56.5/16.00 = 3.53 /1.17 = 3
Empirical
Formula
= NaNO
3
Empirical Formula
When numbers are too far to round, you may need to multiply all values by the same factor to make all numbers whole
What is the empirical formula of a compound that contains
40.6g of calcium and 9.5g of nitrogen?
too far to round
Ca 40.6/40.1 = 1.01 /0.69 = 1.5 × 2 = 3
N 9.5/14.01 = 0.69 /0.69 = 1 × 2 = 2
Empirical
Formula
= Ca
3
N
2 double both numbers to get whole numbers
Molecular Formula
Indicates actual number of atoms of each element in a compound
Multiple of empirical formula
Empirical
Formula
CH
4
Molecular
Formula
C
3
H
12
An empirical formula can be the molecular formula, but the molecular formula is not always the empirical formula
Molecular Formula
If the molecular mass is known, you can solve for the molecular formula
The molar mass of a compound with empirical formula of CH
2
O is 180.12 g/mol. What is the molecular formula of this compound?
Molar mass CH
2
180.12
30.02
Molecular Formula = CH
2
O × 6 = C
6
H
12
O
6