Unit 7 Measurements scientific notation and
advertisement

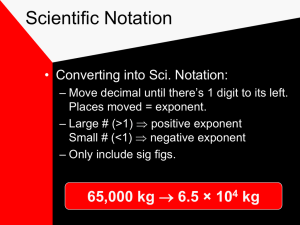
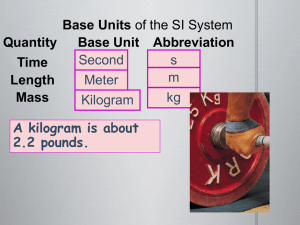
Measurements in Chemistry Scientific notation and Significant Figures SCIENTIFIC NOTATION We commonly measure objects that are many times larger or smaller than our standard of comparison Writing large numbers of zeros is tricky and confusing the sun’s diameter is 1,392,000,000 m an atom’s average diameter is 0.000 000 000 3 m Scientific notation expresses a number as the product of two factors: M x 10n 1 ≤ M 10 and n is an integer Numbers > one have a positive exponent Numbers < one have a negative exponent. Ex. 120000 = 1.2 X 105 0.000054 = 54 x 10-4 (incorrect) 0.000054 = 5.4 x10-5 (CORRECT) Writing a Number In Scientific Notation 0.00012340 1 Locate the Decimal Point 0.00012340 2 Move the decimal point to the right of the first non-zero digit from the left 1.2340 3 Multiply the new number by 10n where n is the number of places you moved the decimal pt. 1.2340 x 104 4 if the number is 1, n is +; if the number is < 1, n is 1.2340 x 10-4 Writing a Number In Scientific Notation 154000 1 Locate the Decimal Point 154000. 2 Move the decimal point to the right of the first nonzero digit from the left 1 . 54000 Multiply the new number by 10n where n is the number of places you moved the decimal pt. 1 . 54 X 105 if the number is 1, n is +; if the number is < 1, n is - Writing a Number in Standard Form 1.234 x 10-6 since exponent is -6, make the number smaller by moving the decimal point to the left 6 places if you run out of digits, add zeros 000 001.234 0.000 001 234 Learning Check Change to scientific notation. 12,340 = 0.369 = 0.008 = 2,050,000,000 = Learning Check Change to scientific notation. 12,340 = 1.234 x 104 –1 3.69 x 10 0.369 = 0.008 = 8 x 10–3 2,050,000,000 = 2.05 x 109 Using the Exponent Key on a Calculator EE EXP EE or EXP means “times 10 to the…” 23: How How to type out 6.02 x 1023 6 0 . 2 EE 2 3 Don’t do it like this… 6 WRONG! 0 . yx 2 2 3 WRONG! …or like this… 6 . 0 2 x 1 …or like this: 6 . 0 EE 2 3 TOO MUCH WORK. 0 2 x 1 0 yx 2 3 1.2 x 105 Example: 2.8 x 1013 Type this calculation in like this: 1 . 2 EE 5 2 . 8 EE 1 Calculator gives… or… This is NOT written… But instead is written… 3 4.2857143 –09 4.2857143 E–09 4.3–9 4.3 x 10–9 = Learning Check (we will learn how to round later) 7.5 x 10-6 - 8.7 x 10-4 4.35 x 106 1.23 x 10-3 5.76 x 10-16 9.86 x 10-4 8.8 x 10 3.3 x 10 11 11 6.022 x 1023 - 5.1 x 10-8 Learning Check (we will learn how to round later) 7.5 x 10-6 - 8.7 x 10-4 = -6.525 x 10-9 4.35 x 106 1.23 x 10-3 = 5.3505 x 103 or 5350.5 5.76 x 10-16 9.86 x 10-4 = 5.84178499 x 10-13 8.8 x 10 3.3 x 10 = 2.904 x 1023 11 11 6.022 x 1023 - 5.1 x 10-8 = -3.07122 x 1016 Classwork: p 948 #1-3 What is a Measurement? quantitative observation They have a number and a unit (indicates what your are measuring Ex. m, s, C) Accuracy and Precision Figure 2.29a -Accuracy refers to how close the measured result is to the true value -Precision refers to closeness to another series of measurement made on the same object- repeatability SIGNIFICANT FIGURES (DIGITS) Our measurements should reflect the precision of the instrument we used. 2.5 cm 2.51 cm INCORRECT: 2.50154 cm Why? I can’t read all this numbers with my instrument. The significant figures (sig figs) of a measurement are those digits known with certainty (read directly from the instrument) plus the last digit which is estimated. 2.34 cm Counting Significant Figures in Measurements 1. All nonzero digits are significant Ex. 725 cm 5.8 C has 3 sig. fig. has 2 sig. fig. 2. Zeros between nonzero digits are significant. Ex. 5.04s 7008 L has 3 sig. fig. has 4 sig. fig. 3. Zeros at the end of a number AND to the right of the decimal point are significant. Ex. 74.50 g has 4 sig figs 208.250 km has 6 sig figs 74, 000 hours has 2 sig figs 200 m has 1 sig fig 4. Zeros at the beginning of numbers are not significant. Ex. 0.0052mm has 2 sig fig 0.00000007800 g has 4 sig. fig. 5. Counted values (18 students) and numbers in defined relationships (1m=100cm) have unlimited number of significant figures and never affect the number of significant figures in the results of a calculation. Zeros at the end of a number without a written decimal point are ambiguous and should be avoided by using scientific notation Ex. if 150m has 2 sig. figs. then 1.5 x 102 m A decimal point is written intentionally to indicate the zero is significant. Ex. 150.m has 3 sig. figs. Learning Check: Determining the Number of Significant Figures in a Number How many sig figs are in each of the measurements? 0.0035 m 1.080 km 2371 C 2.97 × 105 kg 1 dozen = 12 100,000 days 100,000. days Learning Check: Determining the Number of Significant Figures in a Number How many sig figs are in each of the measurements? 0.0035 m 1.080 km 2371 C 2.97 × 105 kg 1 dozen = 12 100,000 days 100,000. days 2 sig figs 4 sig figs 4 sig figs 3 sig. figs. – only decimal parts count unlimited 1 sig figs. 6 sig figs. Rounding using Significant Figures Multiplication and Division with Significant Figures when multiplying or dividing measurements with significant figures, the result has the same number of significant figures as the measurement with the fewest number of significant figures 5.02m × 89.665m × 0.10m= 45.0118m3 = 45m3 3 sig. figs. 5 sig. figs. 2 sig. figs. 2 sig. figs. 5.892 m ÷ 6.10s = 0.96590 m/s = 0.966 m/s 4 sig. figs. 3 sig. figs. 3 sig. figs. Rounding when rounding to the correct number of significant figures, if the number after the place of the last significant figure is 1. 0 to 4, round down 2. 5 to 9, round up Rounding Ex: round to 2 significant figures 0.0234 rounds to 0.023 or 2.3 × 10-2 because the 3 is where the last sig. fig. will be and the number after it is 4 or less 0.0237 rounds to 0.024 or 2.4 × 10-2 because the 3 is where the last sig. fig. will be and the number after it is 5 or greater 0.0299865 rounds to 0.030 or 3.0 × 10-2 because the 9 is where the last sig. fig. will be and the number after it is greater than 5 Learning Check: Determine the Correct Number of Significant Figures for each Calculation and Round and Report the Result 1. 1.01 × 0.12 × 53.51 ÷ 96 = 2. 56.55 × 0.920 ÷ 34.2585 = Learning Check: Determine the Correct Number of Significant Figures for each Calculation and Round and Report the Result 1. 1.01 × 0.12 × 53.51 ÷ 96 = 0.067556 2. 56.55 × 0.920 ÷ 34.2585 = 1.51863 Determine the Correct Number of Sig. Figs. for each Calculation and Round the Result 1. 1.01 × 0.12 × 53.51 ÷ 96 = 0.067556 = 0.068 3 sf 2 sf 4 sf 2 sf result should have 2 sf 7 is in place of last sig. fig., number after is 5 or greater, so round up 2. 56.55 × 0.920 ÷ 34.2585 = 1.51863 = 1.52 4 sf 3 sf 6 sf result should have 3 sf 1 is in place of last sig. fig., number after is 5 or greater, so round up Addition and Subtraction with Significant Figures when adding or subtracting measurements with significant figures, the result has the same number of decimal places as the measurement with the fewest number of decimal places 5.74 + 0.823 + 2.651 = 9.214 = 9.21 2 dec. pl. 3 dec. pl. 3 dec. pl. 2 dec. pl. 4.8 1 dec. pl 3.965 = 3 dec. pl. 0.835 = 0.8 1 dec. pl. Determine the Correct Number of Sig. Figs. for each Calculation and Round Result 1. 0.987 + 125.1 – 1.22 = 124.867 2. 0.764 – 3.449 – 5.98 = -8.664 Determine the Correct Number of Sig. Figs. And Round the Result 1. 0.987 + 125.1 – 1.22 = 124.867 = 124.9 3 dp 1 dp 2 dp result should have 1 dp 2. 0.764 – 3.449 – 5.98 = -8.664 3 dp 3 dp 2 dp 8 is in place of last sig. fig., number after is 5 or greater, so round up result should have 2 dp = -8.66 6 is in place of last sig. fig., number after is 4 or less, so round down Both Multiplication/Division and Addition/Subtraction with Sig. Figs. when doing different kinds of operations with measurements with significant figures, do whatever is in parentheses first, find the number of significant figures in the intermediate answer, then do the remaining steps 3.489 × (5.67 – 2.3) = 2 dp 1 dp 3.489 × 3.37 = 3.489 × 3.4 = 12 4 sf 2 sf 2 sf Classwork: p951 #4 p953 #5-6