Ch.3 - Interactions and Implications
advertisement

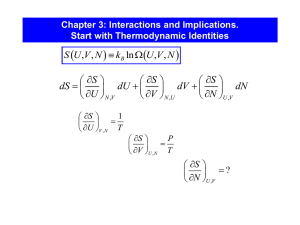
Chapter 3 Interactions and Implications Entropy Entropy Let’s show that the derivative of entropy with respect to energy is temperature for the Einstein solid. Let’s show that the derivative of entropy with respect to energy is temperature for the monatomic ideal gas. Let’s prove the 0th law of thermodynamics. An example with the Einstein Solid Heat Capacity, Entropy, Third Law • • • • • Calculate W Calculate S = kB ln(W) Calculate dS/dU = 1/T Solve for U(T) Easy Cv = dU/dT Difficult to impossible Easy Easy Easy – we’ll see a better way in Ch . 6 w/o needing W Heat capacity of aluminum Let’s calculate the entropy changes in our heat capacity experiment. Heat Capacity, Entropy, Third Law What were the entropy changes in the water and aluminum? DS = Sf – Si = C ln(Tf/Ti) Heat Capacity, Entropy, Third Law As a system approaches absolute zero temperature, all processes within the system cease, and the entropy approaches a minimum. The Third Law It doesn’t get that cold. limS 0 T 0 limCV 0 T 0 As a system approaches absolute zero temperature, all processes within the system cease, and the entropy approaches a minimum. Stars and Black Holes modeled as orbiting particles m1 Show the potential energy is equal to negative 2 times the kinetic energy. r r m2 Stars and Black Holes modeled as orbiting particles m1 Show the potential energy is equal to negative 2 times the kinetic energy. r r m2 Stars and Black Holes modeled as orbiting particles m1 What happens when energy is added? If modeled as an ideal gas what is the total energy and heat capacity in terms of T? r r m2 Stars and Black Holes modeled as orbiting particles m1 Use dimensional analysis to argue potential energy should be of order -GM2/R. Estimate the number of particles and temperature of our sun. r r m2 Stars and Black Holes modeled as orbiting particles m1 What is the entropy of our sun? r r m2 Black Holes What is the entropy a solar mass black hole? Black Holes What are the entropy and temperature a solar mass black hole? S U Mechanical Equilibrium Mechanical Equilibrium Mechanical Equilibrium Diffusive Equilibrium Diffusive Equilibrium Chemical potential describes how particles move. The Thermodynamic Identity Diffusive Equilibrium Chemical potential describes how particles move. Diffusive Equilibrium Chemical potential describes how particles move. Diffusive Equilibrium Chemical potential describes how particles move. Diffusive Equilibrium Chemical potential describes how particles move. Entropy http://www.youtube.com/watch?v=dBXL93984cQ The Thermodynamic Identity The Thermodynamic Identity Paramagnet Paramagnet U +mB Down, antiparallel 0 -mB Up, parallel Paramagnet Paramagnet Paramagnet • M and U only differ by B Nuclear Magnetic Resonance wo = 900 MHz B = 21.2 T wo = g B g = 42.4 (for protons) Nuclear Magnetic Resonance B S Inversion recovery Quickly reverse magnetic field NmB Low U (negative stable) Work on system lowers entropy but it will absorb any available energy to try and slide towards max S U M B NmB NmB High U (positive unstable) Work on system lowers entropy but it will absorb any available energy to try and slide towards max S t Analytical Paramagnet Analytical Paramagnet Analytical Paramagnet Analytical Paramagnet Paramagnet Paramagnet Properties Paramagnet Properties Paramagnet Heat Capacity Magnetic Energies