Chapter 2 Lecture
advertisement

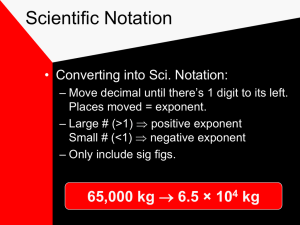
Chapter 2 Measurements 1 CHAPTER OUTLINE Scientific Notation Error in Measurements Significant Figures Rounding Off Numbers SI Units Conversion of Factors Conversion of Units Volume & Density 2 What is a Measurement? quantitative observation comparison to an agreed upon standard every measurement has a number and a unit A Measurement the unit tells you what standard you are comparing your object to the number tells you 1. what multiple of the standard the object measures 2. the uncertainty in the measurement Scientists have measured the average global temperature rise over the past century to be 0.6°C °C tells you that the temperature is being compared to the Celsius temperature scale 0.6 tells you that 1. the average temperature rise is 0.6 times the standard unit 2. the uncertainty in the measurement is such that we know the measurement is between 0.5 and 0.7°C SCIENTIFIC NOTATION Scientific Notation is a convenient way to express very large or very small quantities. Its general form is A x 10n coefficient n = exponent 1 A < 10 6 SCIENTIFIC NOTATION To convert from decimal to scientific notation: Move the point by in the original number Follow thedecimal new number a multiplication signso that 10 it iswith located after the(power). first nonzero digit. and an exponent The exponent is equal to the number of places that the decimal point was shifted. 75000000 7.5 x 10 7 7 Scientific Notation: Writing Large and Small Numbers A positive exponent means 1 multiplied by 10 n times. A negative exponent (–n) means 1 divided by 10 n times. SCIENTIFIC NOTATION For numbers smaller than 1, the decimal moves to the left and the power becomes negative. 0 00642 6.42 x 10 3 9 Examples: 1. Write 6419 in scientific notation. decimal after first nonzero digit power of 10 64.19x10 641.9x10 6419. 6419 6.419 x 10213 10 Examples: 2. Write 0.000654 in scientific notation. decimal after first nonzero digit power of 10 -1 -2 -3 -4 0.000654 0.00654 x 10 0.0654 0.654 10 6.54 xx10 11 CALCULATIONS WITH SCIENTIFIC NOTATION To perform multiplication or division with scientific notation: 1. Change numbers to exponential form. 2. Multiply or divide coefficients. 3. Add exponents if multiplying, or subtract exponents if dividing. 4. If needed, reconstruct answer in standard exponential form. 12 Example 1: Multiply 30,000 by 600,000 Convert Multiply Reconstruct Add to exponential exponents coefficients answerform (3 x 104) (6 x 105) = 18 x 10 9 1.8 x 1010 13 Example 2: Divided 30,000 by 0.006 Convert Subtract Reconstruct Divide to exponential coefficients exponents answerform 4 – (-3) (3 x 104) (6 x 10-3) = 0.5 x 10 7 5 x 106 14 Follow-up Problems: (5.5x103)(3.1x105) = 17.05x108 = 1.7x109 (9.7x1014)(4.3x1020) = 41.71x106 = 4.2x105 6 2.6x10 4 0.4483x10 = 5.8x102 1.7x10 8.2x10 = 4.5x103 5 8 = 0.2073x103 = 2.1x102 (3.7x106)(4.0x108) = 14.8x102 = 1.5x103 15 Follow-up Problems: (8.75x1014)(3.6x108) = 31.5x1022 = 3.2x1023 28 1.48x10 41 = 0.2041x10 7.25x1013 = 2.04x1042 16 ACCURACY & PRECISION Precision is the reproducibility of a measurement Is this compared to other similar measurements. measurement Precision describes how close measurements are precise? to one another. Precision is affected by random errors. Avg mass = 3.12± 0.01 g This measurement has high precision because the deviation of multiple trials is small. 17 ACCURACY & PRECISION Accuracy is the closeness of a measurement to an Is this measurement accepted value (external standard). accurate? Accuracy describes how true a measurement is. Accuracy is affected by systematic errors. Avg mass = 3.12± 0.01 g True mass = 3.03 g cannothas below determined ThisAccuracy measurement accuracywithout because of the accepted the knowledge deviation from true value isvalue. large. 18 ACCURACY & PRECISION Poor precision Good precision Good accuracy Poor accuracy Good precision Poor precision Good accuracy Poor accuracy 19 ACCURACY & PRECISION Two types of error can affect measurements: Systematic errors: those errors that are controllable, and cause measurements to be either higher or lower than the actual value. Random errors: those errors that are uncontrollable, and cause measurements to be both higher and lower than the average value. 20 ERROR IN MEASUREMENTS Two kinds of numbers are used in science: Counted or defined: exact numbers; have no uncertainty Measured: are subject to error; have uncertainty Every measurement has uncertainty because of instrument limitations and human error. 21 ERROR IN MEASUREMENTS certain certain 8.65 8.6 uncertain uncertain What Theislast thisdigit measurement? in any measurement is the What is this measurement? estimated one. 22 RECORDING MEASUREMENTS TO THE PROPER NO OF DIGITS What is the correct value for each measurement? a) 28ml (1 certain, 1 uncertain) b) 28.2ml (2 certain, 1 uncertain) c) 28.31ml (3 certain, 1 uncertain) 23 SIGNIFICANT FIGURES RULES 1. Significant figures figures are arecertain used toand determine uncertain All non-zero digitsrules arethe significant. which digits in digits a measurement. are significant and which are not. 2. All sandwiched zeros are significant. 3. Leading zeros (before or after a decimal) are NOT significant. 4. Trailing zeros (after a decimal) are significant. 0 . 0 0 4 0 0 4 5 0 0 24 Examples: Determine the number of significant figures in each of the following measurements. 461 cm 3 sig figs 1025 g 4 sig figs 0.705 mL 3 sig figs 93.500 g 5 sig figs 0.006 m 1 sig fig 5500 km 2 sig figs 25 ROUNDING OFF NUMBERS If rounded digit is less than 5, the digit is dropped. 51.234 Round to 3 sig figs 1.875377 Less than 5Round to 4 sig figs Less than 5 26 ROUNDING OFF NUMBERS If rounded digit is equal to or more than 5, the digit is increased by 1. 4 51.369 Round to 3 sig figs 1 5.4505 More than Round 5 to 4 sig figs Equal to 5 27 SIGNIFICANT FIGURES & CALCULATIONS The results of a calculation cannot be more precise than the least precise measurement. In multiplication or division, the answer must contain the same number of significant figures as in the measurement that has the least number of significant figures. For addition and subtraction, the answer must have the same number of decimal places as there are in the measurement with the fewest decimal places. 28 MULTIPLICATION & DIVISION 3 sig figs 4 sig figs Calculator answer (9.2)(6.80)(0.3744) = 23.4225 2 sig figs The answer should have two significant figures because 9.2 is the number with the fewest significant figures. The correct answer is 23 29 ADDITION & SUBTRACTION Add 83.5 and 23.28 Least precise number Calculator answer Correct answer 83.5 23.28 106.78 106.8 30 Example 1: 5.008 + 16.2 + 13.48 = 34.688 34.7 Least precise number Round to 31 Example 2: 3 sig figs 3.15 x 1.53 = 6.1788 0.78 6.2 2 sig figs Round to 32 SI UNITS Measurements are made by scientists to determine size, length and other properties of matter. For measurements to be useful, a measurement standard must be used. A standard is an exact quantity that people agree to use for comparison. SI is the standard system of measurement used worldwide by scientists. 33 SI (METRIC) BASE UNITS Quantity Measured Length Metric Units Meter m English Units yd Symbol Mass Kilogram kg lb Time Seconds s s Temperature Kelvin K F Mole mol mol Amount of substance 34 Basic Units of Measurement The kilogram is a measure of mass, which is different from weight. The mass of an object is a measure of the quantity of matter within it. The weight of an object is a measure of the gravitational pull on that matter. Consequently, weight depends on gravity while mass does not. Derived Units A derived unit is formed from other units. Many units of volume, a measure of space, are derived units. Any unit of length, when cubed (raised to the third power), becomes a unit of volume. Cubic meters (m3), cubic centimeters (cm3), and cubic millimeters (mm3) are all units of volume. DERIVED UNITS In addition to the base units, several derived units are commonly used in SI system. Quantity Measured Units Symbol Volume Liter L Density grams/cc g/cm3 37 SI PREFIXES The Common SI system prefixes of units are used is easy with to the use base because units it is to SI Prefixes indicate multiple ten that the unit represents. based onthe multiples ofof ten. Prefixes mega- Symbol M kilocentimilli- k c m micro- Multiplying factor 1,000,000 106 1000 0.01 0.001 103 10-2 10-3 0.000,001 10-6 38 SI UNITS & PREFIXES SI system used a common set of prefixes for use with the base units. 106 103 Base Unit 103 106 10 10 10 10 10 10 10 10 10 10 10 10 micro Smaller units milli deci centi kilo mega Larger units 39 SI CONVERSION FACTORS 106 10 10 micro Base Unit 103 10 10 milli 10 10 deci centi 10 103 10 10 106 10 kilo 10 10 mega 103 mm or 1 mm = 103 m 1 mm = 103 m or 1 m = 103 mm 1m= 40 SI PREFIXES How many cm mmare areininaakm? cm? 100000 10x10x10x10x10 10 or 105 41 Prefix Multipliers Choose the prefix multiplier that is most convenient for a particular measurement. Pick a unit similar in size to (or smaller than) the quantity you are measuring. A short chemical bond is about 1.2 × 10–10 m. Which prefix multiplier should you use? The most convenient one is probably the picometer. Chemical bonds measure about 120 pm. CONVERSION FACTORS Many problems in chemistry and related fields require a change of units. Any unit can be converted into another by use of the appropriate conversion factor. Any equality in units can be written inMetric-Metric the form of a Factor fraction called a conversion factor. For example: Equality Conversion Factors 1 m = 100 cm 1m 100 cm or 100 cm 1m 43 CONVERSION FACTORS Metric-English Factor Equality 1 kg = 2.20 lb Conversion Factors 1 kg 2.20 lb or 2.20 lb 1 kg Sometimes a conversion factor is given Percentage as a percentage. For example: Factor Percent quantity: Conversion Factors 18% body fat by mass 18 kg body fat 100 kg body mass or 100 kg body mass 18 kg body fat 44 CONVERSION OF UNITS Problems involving conversion of units and other chemistry problems can be solved using the following step-wise method: 4. 2. 3. Write Set Planupathe the sequence conversion problem of steps by factor arranging to convert forand each cancelling the units initial change units unit in in tothe the 1. Determine the intial unit given the final unit needed. final unit. your numerator plan. and denominator of the steps involved. beginning unit x final unit = final unit beginning unit Conversion factor 45 Example 1: Convert 164 lb to kg (1 kg = 2.20 lb) Step 1: Step 2: Step 3: Step 4: Given: 164 lb lb 1 kg 2.20 lb Need: kg Metric-English factor or kg 2.20 lb 1 kg 1 kg 164 lb x = 74.5 kg 2.20 lb 46 Example 2: The thickness of a book is 2.5 cm. What is this measurement in mm? Step 1: Given: 2.5 cm Step 2: cm Step 3: 1 cm 10 mm Step 4: Need: mm Metric-Metric factor or mm 10 mm 1 cm 10 mm 2.5 cm x = 25 mm 1 cm 47 Example 3: How many centimeters are in 2.0 ft? (1 in=2.54 cm) Step 1: Given: 2.0 ft Step 2: ft Step 3: Step 4: English-English factor 1 ft 12 in and Need: cm in Metric-English factor cm 1 in 2.54 cm 12 in 2.54 cm 61 cm cm = 60.96 2.0 ft x x 1 in 1 ft 48 Example 4: Bronze is 80.0% by mass copper and 20.0% by mass tin. A sculptor is preparing to case a figure that requires 1.75 lb of bronze. How many grams of copper are needed for the brass figure (1lb = 454g)? Step 1: Step 2: Given: 1.75 lb bronze lb brz English-Metric factor g brz Need: g of copper Percentage factor g Cu 49 Example 4: Step 3: Step 4: 1 lb 454 g 1.75 lb brz x and 454 g 1 lb 80.0 g Cu 100 g brz 80.0 g Cu x == 635.6 636 g 100 g brz 50 VOLUME Volume is the amount of space an object occupies. Common units are cm3 or liter (L) and milliliter (mL). 1 L = 1000 mL 1 mL = 1 cm3 51 VOLUME Volume of various regular shapes can be calculated as follows: Cube V = s x s x s Cylinder V = π x r2 x h Rect. V = l x w x h Sphere V =4/3 πr3 52 DENSITY Density is mass per unit volume of a material. Common units are g/cm3 (solids) or g/mL (liquids). Density is directly Density is indirectly related to the related to the mass of an object. volume of an object. Which has greatest density? 53 Example 1: A copper sample has a mass of 44.65 g and a volume of 5.0 mL. What is the density of copper? m = 44.65 g m 44.65 g d= = = 8.9 8.93g/mL g/mL v 5.0 mL v = 5.0 mL d = ??? Round to 2 sig figs 54 Example 2: A silver bar with a volume of 28.0 cm3 has a mass of 294 g. What is the density of this bar? m = 294 g m 294 g d= = = 10.5 g/mL v 28.0 mL v = 28.0 mL d = ??? 3 sig figs 55 Example 3: If the density of gold is 19.3 g/cm3, how many grams does a 5.00 cm3 nugget weigh? Step 1: Step 2: Step 3: Given: 5.00 cm3 cm3 g density 5.00 cm3 Need: g x 19.3 g 1 cm 3 = 96.5 g 56 Example 4: If the density of milk is 1.04 g/mL, what is the mass of 0.50 qt of milk? (1L = 1.06 qt) Step 1: Step 2: Step 3: Given: 0.5 qt qt English-Metric factor 1L 1.06 qt Need: g L and Metric-Metric factor 103 mL 1L mL and density 1.04 g 1 mL 1 L 103 mL 1.04 g x x Step 4: 0.50 qt x == 490 490.57 g g 1.06 qt 1 mL 1L 57 g Example 5: What volume of mercury has a mass of 60.0 g if its density is 13.6 g/mL? 1 mL 4.41 mL = 60.0 g x 13.6 g inverse of density 58 IS UNIT CONVERSION IMPORTANT? Further In 1999 investigation Mars Climateshowed orbiter was lost space that engineers at in Lockheed becausewhich engineers Martin, builtfailed the to make a simple conversion aircraft, calculated from Englishmeasurements units to metricin navigational units, an embarrassing lapse English units. When NASA’s thatengineers sent the $125 million JPL received the craft they fatally close tothe the data, assumed Martian surface. information was in metric units, causing the confusion. 59 THE END 60