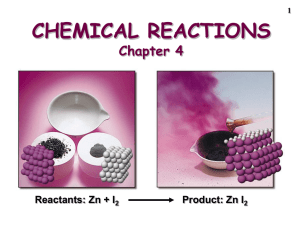
Chapter 3 Stoichiometry
advertisement
Chapter 3 Stoichiometry Stoichiometry: The study of quantities of materials consumed and produced in chemical reactions Atomic Masses: Are determined by comparing with 12C (carbon-12 scale). By definition, carbon-12 is assigned a mass of exactly 12 atomic mass units (amu) and the masses of all other atoms are given relative to this standard Measurement of Atomic Masses: Mass spectrometer is used to measure the atomic masses. Charged particles are bent in a magnetic field and the deflection depends on the masses which causes the ions to separate. A comparison of the positions where the ions hit the detector plate gives very accurate values of their relative masses Schematic Diagram of a Mass Spectrometer Atomic Masses Isotopes: Elements occur in nature as mixtures of isotopes. Carbon = 98.89 % 12C & 1.11 % 13C Carbon atomic mass = (0.9889)(12 amu) + (0.0111)(13.0034) = 12.01 amu Average atomic mass = [(relative abundance of each isotope) x (the mass of each isotope)] Lithium consists of: 7.50 % 6Li which has an accurate weight of 6.015121 amu and 92.50 % 7Li which has an accurate weight of 7.016003 amu. Av. At. Mass? Av. At. Mass = (0.0750) x (6.015121 amu/atom) + (0.9250) x (7.016003 amu/atom) = (0.4511) amu + (6.4898) amu = 6.941 amu Neon Gas Mass Spectrum of Natural Copper The Mole Mole: The amount of a substance that contains as many entities as there are in exactly 12 g of carbon-12. 1 mole of anything = 6.022 x 1023 units of that thing (Avogadro’s number) The mole is defined as a sample of a natural element with a mass equal to the element’s atomic mass expressed in grams contains 1 mole of atoms. 1 mole of carbon = 12.01g of carbon = 6.022X1023 atoms C How many Li atoms are there in 3.0 g of lithium? 3.0 g Lithium x (1 mol Lithium)/(6.941g) x (6.022 x 1023 atom)/(1mol Lithium) = 2.6 x 1023 atoms of Li. Cobalt is a metal that is added to steel to improve its resistance to corrosion. Calculate both the number of moles in a sample of cobalt containing 5.00 x 1020 atoms and the mass of the sample. 5.00 x 1020 atoms Co x (1 mol Co)/ 6.022 x 1023atoms Co) = 8.30 x 10-4 mol Co 8.30 x 10-4 mol Co x (58.93 g Co)/(1 mol Co) = 4.89 x 10-2 g Co Example How much does 2.0 x 1024 atoms of Ne weigh? 2.0 x 1024 atoms Ne x (1 mol Ne)/(6.022X1023 atoms Ne) x (20.18 g Ne)/(1 mol Ne) = 67g 8.46 g B = ? Moles 8.46 g B x (1 mol B)/(10.81 g B) = 0.783 moles of B Molar Mass Molar Mass: A substance’s molar mass is the mass in grams of one mole of the substance Molar mass of a substance is numerically equal to its formula mass Molar mass of CO2 = 12.01 g + 2 x 16.00 g = 44.01 g Molar mass of Al2(SO4)3 — 2 Al = 2 x 26.98 = 53.96; 3 S = 3 x 32.06 = 96.18 12 O = 12 x 16.00 = 192.0; Total = 342.14 g 1 mole of Al2(SO4)3 = 342.1 g How many atoms of Al are in 52 g of Al2(SO4)3? 52 g x (1 mol)/(342.1 g) x (6.022 x 1023 molecules)/(1 mol) x (2 Al atom)/(1 molecule) = 1.8 x 1023 atoms Percent Composition of Compounds If the formula of the compound is known, the % composition can be calculated. Elemental % = (mass of element in compound)/mass of compound) x 100 Compute the mass percent of each element in Penicillin F which has the formula C14H20N2SO4. C: 14 mol x 12.01 g/mol = 168.1 g H: 20 mol x 1.008 g/mol = 20.16 g N: 2 mol x 14.01 g/mol = 28.02 g S: 1 mol x 32.07 g/mol = 32.07 g O: 4 mol x 16.00 g/mol = 64.00 g Example Mass of 1 mol C14H20N2SO4 = 312.4 g % C = (168.1 g)/(312.4 g) x 100 % = 53.81 % % H = (20.16 g)/(312.4 g) x 100 % = 6.453 % % N = (28.02 g)/(312.4 g) x 100 % = 8.969 % % S = (32.07 g)/(312.4 g) x 100 % = 10.27 % % O = (64.00 g)/(312.4 g) x 100% = 20.49 % Determining the Formula of a compound If we know the % composition we can find the formula. Molecular formula = (empirical formula)n where n=integer Empirical formula = CH = simplest formula Molecular formula = (CH)6 = C6H6 For some compounds (like H2O, NaCl, NH3) the empirical and the molecular formulas are the same, for others it is different (empirical formula = CH4, the molecular formula = C2H8) Schematic Diagram of the Combustion Device Used to Analyze Substances for Carbon and Hydrogen How do you find an empirical (simplest) formula? 1. Convert % to grams of each element 2. Convert % grams to moles of atoms 3. Divide by the lowest number of moles 4. If needed adjust for fractions Example: A white powder determined to have 43.64 % P and 56.36 % O (Simplest way is to consider 100 g sample) Step 1: 43.64 % P 43.64 g P 56.36 % O 56.36 g O Step 2: 43.64 g P x (1 mol P)/(30.97 g) = 1.409 mol 56.36 g O x (1 mol O)/(16.00 g) = 3.523 mol Step 3: P1.409/1.409 = 1; O3.523/1.409 = 2.5 Step 4: P1O2.5 x 2 = P2O5 empirical formula What is the true molecular formula? Obtain the empirical formula Compute the mass of the empirical formula Calculate the ratio (integer) of the molar mass/empirical formula mass Multiply the empirical formula subscript by the integer gives the molecular formula For the above compound, molecular weight = 290 g/mole, What is the molecular formula? Empirical weight— 2 P = 2 x 30.97 = 61.94 g 5 O = 5 x 16.00 = 80.00 g Total = 141.94 g 141.9 g N = 290/142 2.04 must be integer 2 Molecular formula = (P2O5 )2 = P4O10 (tetraphosphorus decaoxide) Chemical Equations A chemical change involves a reorganization of the atoms in one or more substances. Chemical equation is a representation of a chemical reaction with the reactants on the left side of an arrow and the products on the right side C2H5OH + 3O2 2CO2 + 3H2O Reactants Products Atoms have been reorganized. Bonds have been broken and new ones have been formed In a chemical reaction, atoms are neither created nor destroyed All atoms present in the reactants must be accounted for among the products (i.e. # atoms need to be same on both sides of the arrow) The above equation is balanced. The Meaning of a Chemical Equation CH4(g) + 2O2(g) CO2(g) + 2H2O(g) The nature of the reactants and products (physical states) The relative numbers of reactants and products (# moles) The relative numbers of the reactants and products in a reaction are indicated by the coefficients in the balanced equation 1 mole of methane reacts with 2 moles of oxygen to produce 1 mole of carbon dioxide and 2 moles of water 1 molecule CH4 + 2 molecule O2 produce 1 molecule CO2 + 2 molecule H2O 16 g CH4 + 2(32 g) O2 produce 44 g CO2 + 2(18 g) H2O 80 g reactants produce 80 g products Balancing Chemical Equations The total number of atoms of any element must be the same on both sides of the chemical equation The identities of the reactants and products must not be changed The formulas of the compounds must never be changed in balancing a chemical equation The subscripts in a formula cannot be changed, nor can atom be added or subtracted from a formula Need to balance the equation by inspection, i.e. by trial and error Writing and Balancing the Equation for a Chemical Reaction Determine the reactants, the products and the physical states Write the unbalanced equation that summarizes the reaction Balance the equation by inspection (NH4)2Cr2O7(s) Cr2O3(s) + N2(g) + H2O(g) 2N, (4x2)H, 2Cr, 70 2Cr, 30, 2N, 2H, 10 (NH4)2Cr2O7(s) Cr2O3(s) + N2(g) + 4 H2O(g) The equation is balanced Stoichiometric Calculations: Amounts of Reactants and Products Balance the equation for the reaction Convert mass to moles Set up appropriate mole ratios from balanced equation Use mole ratios to calculate moles of desired substituent Convert moles to grams, if necessary Example If 2.85 g of NH3 are burned, how much H2O will be formed? 4NH3 + 5O2 4NO + 6H2O 2.85 g NH3 x (1 mol NH3)/(17.04 g) = 0.167 mol NH3 0.167 mol NH3 x (6 mol H2O)/(4 mol NH3) = 0.251 mol H2O 0.251 mol H2O x (18.02 g H2O)/(1 mol H2O) = 4.52 g H2O Combined: 2.85 g NH3 x (1 mol)/(17.04 g) x (6 H2O)/(4 NH3) x (18.02 g)/(1 mol H2O) = 4.52 g H2O Calculations Involving a Limiting Reactant 1. 2. 3. 4. 5. The limiting reactant is the reactant that is consumed first, limiting the amounts of products formed. Steps for Solving Stoichiometry Problems: Write and balance the equation Convert the known masses of substances to moles Determine which reactant is limiting Using the amount of the limiting reactant and the appropriate mole ratios, compute the number of moles of the desired product Convert the moles to grams, using the molar mass Three Different Stoichiometric Mixtures of Methane and Water Mixture of CH4 and H2O Molecules Methane and Water Reacting Hydrogen and Nitrogen React to Form Ammonia According to the Equation N2 + 3H22NH3 Example 18.1g of gaseous NH3 is reacted at high temperature with 90.4g of solid CuO to produce nitrogen gas, solid copper and water vapor. Find the limiting reactant. How many grams of N2 will be formed? 2 NH3 (g) + 3CuO (s) N2 (g) +3Cu (s) + 3H2O (g) 18.1g NH3 X (1 mol NH3 )/(17.03g NH3) = 1.06 mol NH3 90.4g CuO X (1 mol CuO)/(79.55g CuO) = 1.14 mol CuO 1.06 mol NH3 X (3 mol CuO)/(2 mol NH3) = 1.59 mol CuO 1.14 mol CuO is actually present, the amount of CuO is limiting, CuO will run out before NH3 does. Since CuO is limiting reactant, we need to use CuO to calculate the amount of N2 formed. 1.14 mol CuO X (1mol N2)/(3mol CuO) X (28.0g N2)/(1mol N2) = 10.6g N2 ..Continued Percent yield = (Actual yield)/(Theoretical yield) X 100 % Actual yield: Amount of product actually formed Theoretical yield: The amount of product predicted from the reactants If in the above example, 6.63 grams of nitrogen actually produced instead of the predicted 10.6 grams what is the percent yield? Percent yield = (Actual yield)/(Theoretical yield) X 100 % = (6.63g)/(10.6g) X 100 % = 62.5 % Example 68.5kg CO (g) is reacted with 8.60kg H2 (g) to produce methanol. Calculate the theoretical yield of methanol. If 3.57 X 104g CH3OH is actually produced, what is the percent yield of methanol? 2 H2 (g) + CO (g) CH3OH (l) 68.5kg CO X (1000g CO)/(1kg CO) X (1mol CO)/(28.02g CO) = 2.44 X 103 mol CO 8.60kg H2 X (1000g H2)/(1kg H2) X (1mol H2)/(2.016g H2) = 4.27 X 103 mol H2 2.44 X 103 mol CO X (2mol H2)/(1mol CO) = 4.88 X 103 mol H required ..Continued Mol H2 present is less than mol H2 required, so H2 is limiting reagent so we need to use H2 for theoretical yield. 4.27 X 103 mol H2 X (1mol CH3OH)/(2mol H2) X (32.04g CH3OH)/(1mol CH3OH) = 6.84 X 104g CH3OH % yield = (3.57 X 104g CH3OH)/(6.84 X 104g CH3OH) X 100 % = 52.2 % Summary Stoichiometry: Quantities of materials consumed or produced Atomic masses: Based on exactly 12 amu to a 12C atom Mole: A mole is a unit of measure Avogadro’s number: 6.022 X 1023 Molar mass: Mass in grams of one mole Formula of a compound: Empirical formula & Molecular formula Chemical equation: representation of a chemical reaction Meaning of a chemical equation: physical states & #moles Balancing Chemical equation Stoichiometric calculation: mass to mole conversion Limiting reactant: which limits the amount of product formed