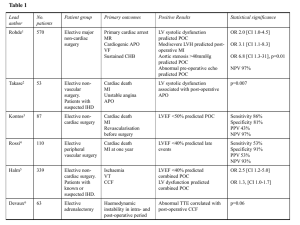
Official journal of Editors in Chief Alberto Zangrillo Roland Hetzer
advertisement

HEART LUNG VESSELS ISSN: 2282-8419 IN THE NEXT ISSUES • CABG in patients with prior PCI • Wedge pressure and natriuretic peptides following mitral valve surgery • Mobile ECMO team: an 8 year experience • TAVI follow-up • Cardiopulmonary bypass temperature: a randomized pediatric trial • Right ventricular dysfunction in VV ECMO and Editors in Chief Alberto Zangrillo Roland Hetzer 2Ԁ FLDOMRXUQDORI Formerly “HSR Proceedings in Intensive Care and Cardiovascular Anesthesia” Vol. 6 · N° 3 · 2014 ED BM D U P XE W DE NO IN ASSOCIATE EDITORS Massimo Antonelli Università Cattolica Sacro Cuore, Policlinico Gemelli, Roma, Italia Antonio Pesenti Università degli Studi di Milano Bicocca, Ospedale San Gerardo, Italia Giovanni Landoni Università Vita-Salute San Raffaele, Milano, Italia Marco Ranieri Università di Torino S. Giovanni Battista Molinette, Torino, Italia Vol. 6 • N° 3 • 2014 September Milan, Italy SECTION EDITORS Q INTENSIVE CARE Ludhmila Abrahao Hajjar University of Sao Paulo, Sao Paulo, Brazil EDITORS IN CHIEF Alberto Zangrillo Università Vita-Salute San Raffaele Milan, Italy Roland Hetzer Deutsches Herzzentrum Berlin, Germany Q ANESTHESIA Fabio Guarracino Azienda Ospedaliera Universitaria Pisana, Pisa, Italia Q VASCULAR SURGERY Roberto Chiesa Università Vita-Salute San Raffaele, Milano, Italia Q CARDIAC SURGERY Ottavio Alfieri Università Vita-Salute San Raffaele, Milano, Italia Official Journal of Roland Hetzer International Cardiothoracic and Vascular Surgery Society Berlin, Germany Endorsed by ITACTA (Italian Association of Cardiothoracic Anaesthesiologists) www.itacta.org Deutsches Herzzentrum Berlin, Germany Q PEDIATRIC CARDIAC SURGERY AND CONGENITAL HEART DISEASES Eva Maria Javier Delmo Walter Children‘s Hospital and Harvard Medical School, Boston, MS, USA; Deutsches Herzzentrum Berlin, Germany Q TRANSPLANTATION AND IMMUNOLOGY Paolo Fiorina Harvard Medical School, Boston, MA, USA Q CARDIOLOGY Giuseppe Biondi-Zoccai Università degli Studi “La Sapienza”, Roma, Italia Q PEDIATRIC CARDIOLOGY Brigitte Stiller Universitaetsklinikum Freiburg, Germany Publisher Q ECHOCARDIOGRAPHY Michele Oppizzi Università Vita-Salute San Raffaele, Milano, Italia Q NEW TECHNOLOGIES Federico Pappalardo Università Vita-Salute San Raffaele, Milano, Italia Q IN HOSPITAL EMERGENCIES Luca Cabrini Edizioni Internazionali srl Divisione EDIMES EDIZIONI MEDICO SCIENTIFICHE - PAVIA Via Riviera 39 - 27100 Pavia Tel. 0382526253 r.a. - Fax 0382423120 E-mail: edint.edimes@tin.it Università Vita-Salute San Raffaele, Milano, Italia Q PEER-TO-PEER COMMUNICATION Michael John Università Vita-Salute San Raffaele, Milano, Italia Q IMAGING Antonio Grimaldi Università Vita-Salute San Raffaele, Milano, Italia Q FUTURE EVENTS George Silvay The Mount Sinai School of Medicine, New York, NY Q SOCIAL MEDIA Laura Pasin Università Vita-Salute San Raffaele, Milano, Italia EDITORS Rinaldo Bellomo Austin Hospital, Melbourne, Australia Friedhelm Beyersdorf Universitätsklinikum Freiburg, Freiburg, Germany Elena Bignami Università Vita-Salute San Raffaele, Milano, Italia Giovanni Borghi Università Vita-Salute San Raffaele, Milano, Italia Editorial Secretariat Lara Sussani Anesthesia and Intensive Care Università Vita-Salute San Raffaele Via Olgettina, 60 - 20132 Milan, Italy Tel. +39 02 26436158 Fax +39 02 26436152 sussani.lara@hsr.it Tiziana Bove www.heartlungandvessels.org IT technical support Ilic Radice Aleph s.r.l. - Milan webmaster@heartlungandvessels.org Director in chief Paolo E. Zoncada Registered at the Milan Tribunal on November 26th 2009 (number 532) The Journal is indexed, among others, in: PubMed and PubMed Central ISSN (ONLINE): 2283-3420 ISSN (PRINTED): 2282-8419 Printed by Jona Srl Paderno Dugnano (MI) Kevin Lobdell Sanger Heart and Vascular Institute, Charlotte, NC, US Carlos Mestres Hospital Clínico, University of Barcelona, Barcelona, Spain Andrea Morelli Università Vita-Salute San Raffaele, Milano, Italia Enrico Camporesi University of South Florida, Tampa, Florida Murali Chakravarthy University Hospital of Split, Split, Croatia Università degli Studi “La Sapienza”, Roma, Italia Daniela Pasero Ospedale San Giovanni Battista, Torino, Italia Gianluca Paternoster Massimo Clementi A.O.R. Ospedale San Carlo, Potenza, Italia Dean, Università Vita-Salute San Raffaele, Milano, Italia Emanuele Piraccini Massimiliano Conte Maria Cecilia Hospital GVM Care & Research, Cotignola (RA), Italia Antonio Corcione Ospedale “G.B. Morgagni-Pierantoni”, Forlì, Italia Jose Luis Pomar Hospital Clínico, University of Barcelona, Barcelona, Spain AORN Dei Colli, V. Monaldi, Napoli Martin Ponschab Laura Corno Trauma Hospital Linz, Linz, Austria Università Vita-Salute San Raffaele, Milano, Italia J. Scott Rankin Remo Daniel Covello Università Vita-Salute San Raffaele, Milano, Italia Michele De Bonis Università Vita-Salute San Raffaele, Milano, Italia Francesco De Simone Università Vita-Salute San Raffaele, Milano, Italia Vanderbilt University, Nashville, Tennessee, USA Marco Ranucci IRCCS Policlinico San Donato, Milano, Italia Zaccaria Ricci Ospedale Pediatrico Bambino Gesù, Roma, Italia Reitze N. Rodseth Ospedale del Cuore, FTGM, Massa, Italia Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa Juergen Ennker Stefano Romagnoli Paolo Del Sarto Mediclin Heart Institute, Lahr, Germany Università di Cagliari, Cagliari, Italia Ospedale Careggi, Firenze, Italia Laura Ruggeri Gian Franco Gensini Università Vita-Salute San Raffaele, Milano, Italia Università degli Studi di Firenze, Italia Anna Mara Scandroglio Ravi Gill Università Vita-Salute San Raffaele, Milano, Italia Massimiliano Greco Università Vita-Salute San Raffaele, Milano, Italia EDIZIONI MEDICO SCIENTIFICHE - PAVIA Via Riviera 39 - 27100 Pavia Tel. 0382526253 r.a. - Fax 0382423120 E-mail: edint.edimes@tin.it McMaster University, Population Health Research Institute, Hamilton, Ontario, Canada Maria Grazia Calabrò University Hospital Southampton NHS Foundation Trust, Southampton, UK Edizioni Internazionali srl Divisione EDIMES Yannick Le Manach Julije Mestrovic Gabriele Finco Publisher Azienda Ospedaliero-Universitaria Careggi, Firenze, Italia Università Vita-Salute San Raffaele, Milano, Italia Fortis Hospitals, Bangalore, India WEB Site Chiara Lazzeri Yoshiro Hayashi Kameda Medical Center, Kamogawa, Chiba, Japan The University of Queensland, Brisbane, Australia Luca Severi Azienda Ospedaliera San Camillo Forlanini, Roma, Italia Andrea Szekely Semmelweis University, Budapest, Hungary Luigi Tritapepe Università degli Studi “La Sapienza”, Roma, Italia James L. Januzzi Emiliano Vitalini Harvard University, Massachusetts General Hospital, US Ospedale San Camillo Forlanini, Roma, Italia CONTENTS Q INVITED EDITORIAL A comprehensive approach to refractory cardiac arrest: saving more lives one way or another ................................................................................................................................................................... 149 I. Ortega-Deballon, E. De La Plaza-Horche Q EXPERT OPINION A simplified minimally invasive approach to mitral valve surgery optimal access under direct vision .............................................................................................................................................................................. 152 A. Amiri, E.M. Delmo Walter, R. Hetzer Q REVIEW ARTICLE Acute right heart syndrome in the critically ill patient ................................................................................................... 157 V. Zochios, N. Jones Q ORIGINAL ARTICLE Atrial fibrillation after isolated coronary surgery. Incidence, long term effects and relation with operative technique ........................................................ 171 ................................................................................. 180 C. Rostagno, C. Blanzola, F. Pinelli, A. Rossi, E. Carone, P.L. Stefàno Acute myocardial infarction associated to DPP-4 inhibitors J.P.L. Nunes, J.D. Rodrigues, F. Melão Agglutinins and cardiac surgery: a web based survey of cardiac anaesthetic practice; questions raised and possible solutions ..................................... 187 S. Shah, H. Gilliland, G. Benson Direct comparison between cerebral oximetry by INVOSTM and EQUANOXTM during cardiac surgery: a pilot study ............................................................................................. 197 A. Pisano, N. Galdieri, T.P. Iovino, M. Angelone, A. Corcione Q CASE REPORT Life threatening tension pneumothorax during cardiac surgery. A case report ........................................................................................................................................................................................................................................................... 204 A. Jain, D. Arora, R. Juneja, Y. Mehta, N. Trehan Q IMAGES IN MEDICINE Coronary to extra-cardiac anastomosis ............................................................................................................................................................ 208 A. Mohsen, J. Loughran, S. Ikram Internal thoracic vein: friend or foe?........................................................................................................................................................................ 210 A. Roubelakis, D. Karangelis, S.K. Ohri Q FUTURE EVENTS ..........................................................................................................................................................................................................................................213 147 Invited Editorial Heart, Lung and Vessels. 2014; 6(3): 149-151 A comprehensive approach to refractory cardiac arrest: saving more lives one way or another I. Ortega-Deballon1,2,3, E. De La Plaza-Horche3 1 McGill University Health Center, Montreal Children’s Hospital and Centre de Prélèvement d’Organes, Hôpital du Sacré-Coeur de Montréal, Québec, Canada; 2Faculty of Medicine and Health Sciences, University of Alcalá de Henares, Madrid, Spain; 3Helicopter and Ambulance Emergency Medical Services SUMMA 112, Madrid, Spain According with the last updated guidelines on resuscitation, the underlying cause of cardiac arrest (CA) should be identified, treated and, if possible, reversed with different strategies but a common target: to increase long-term survival with good neurologic recovery. At the same time, some countries have implemented protocols for donation after considering the irreversibility of cardiac arrest and the failure of resuscitation attempts. Both strategies are complementary and should coexist. Thus, we would be able to go beyond the refractory CA firstly and, if not indicated or unsuccessful, we could increase the organ donation pool after confirming irreversibility. International recommendations on resuscitation (1) highlight the importance of high-quality cardiopulmonary resuscitation (CPR) based on minimal interruptions, focused on determining the cause of the cardiac arrest and offering, as early as possible, etiological treatment of potential reversible causes. Several pioneering protocols have been developed throughout the world in order to provide a multidisciplinary approach to the out-of-hospital (OHCA) and in-hospital cardiac arrest (IHCA). Moreover, international programs including non-conventional resuscitation procedures (NCRPs) have been set up (2-7). Corresponding author: Ivan Ortega-Deballon Research Associate Critical Care Division Montreal Children’s Hospital 2300, rue tupper. Montreal Quebec. Canada. H3H 1P3 e-mail: iviortega@gmail.com Heart, Lung and Vessels. 2014, Vol. 6 149 I. Ortega-Deballon, et al. 150 Such an approach to refractory CA incudes: 1. High-quality CPR with minimal interruptions: ongoing CPR during transportation to the hospital through automated chest compressors and ventilations, possible administration of thrombolytics in pulmonary embolism (1), and use of therapeutic mild hypothermia (3,7). 2. Management of IHCA, or incoming patient with OHCA undergoing CPR: percutaneous coronary intervention during CPR in coronary artery disease, or ECLS followed by thrombolysis, placement of an intra-aortic balloon pump, or therapeutic mild hypothermia (2-7). The conclusions of published studies highlight in the need of validating a predictive model, to establish teams trained in the procedures, and to avoid delays in the initiation of the ECLS technique after admission of the patient to the hospital (minimizing the so-called low-flow period) (2,4-7). Some countries are a reference in programs for uncontrolled donation after circulatory death (uDCD) (9-12): a patient who has an unexpected OHCA, and who fails to CPR attempts is transferred with continuing thoracic compressions and ventilation with the sole aim of preserving the organs (12). Thus, is driven to a hospital with the capacity to receive this type of donor, rather than to the hospital able to treat the underlying cause of CA, when possible (9-12). The family, if not at the scene are asked by police to go to the hospital and there, they receive notification of the death of their relation. Transplant coordinators then ask them for authorization to the organ retrieval. At this time, the deceased person is already in the operating room and has been connected to an organ preserving system. This type of uDCD program provides 10% of all deceased donors in Spain (40% in Madrid region) (12). There are evident similarities between the human, technical and logistical means made available for NCRPs and those required by uDCD programs (9-11). We, obviously, support the need to obtain organs for donation, and the uDCD programs are essential for this laudable purpose (11). However, priorities should be clear and transparent: inclusion in the uDCD program should be considered only after benefiting patients of NCRPs, if they were eligible for it (9-11). Moreover, recent protocols even show that joining both strategies, not only survival outcomes rate increases, but also graft outcomes (3,7). An unexplored path to achieve both these goals might be to implement a comprehensive management of OHCA that includes two options: ‘Non-conventional resuscitation procedures option’ in selected patients, focused on the reversible underlying primary cause of OHCA (option 1) or a protocol for uDCD (option 2) if the ‘ongoing CPR option’ is not indicated or judged futile, after conventional resuscitation attempts have been provided case-by-case. To implement such a protocol requires to build a bridge linking prehospital and hospital settings. Thus, by trying to save hopeless patients’ lives, and when this is not possible, by increasing organ availability for transplantation, we will be providing excellent care to patients suffering refractory cardiac arrest on the field, and saving more lives by one or other way: firstly, through highquality resuscitation, and secondly, when really impossible even after the best attempts, retaining donation and transplantation options after declaring death. Heart, Lung and Vessels. 2014, Vol. 6 A comprehensive approach to refractory cardiac arrest REFERENCES 1. Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, Deakin C, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 2010; 81: 1219-76. 2. Sunde K. Experimental and clinical use of ongoing mechanical cardiopulmonary resuscitation during angiography and percutaneous coronary intervention. Crit Care Med 2008; 36(11 Suppl): S405-8. 3. Fagnoul D, Taccone FS, Belhaj A, Rondelet B, Argacha JF, Vincent JL, et al. Extracorporeal life support associated with hypothermia and normoxemia in refractory cardiac arrest. Resuscitation 2013; 84: 1519-24. 4. Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008; 372: 554-61. 5. Lazzeri C, Bernardo P, Sori A, Innocenti L, Stefano P, Peris A, et al. Venous-arterial extracorporeal membrane oxigenation for refractory cardiac arrest: a clinical challenge. Eur Heart J Acute Cardiovasc Care. 2013; 2: 118-26. 6. Adnet F, Baud F, Cariou A, Carli P, Combes A, Devictor D, et al. Guidelines for indications for the use of extracorporeal life support in refractory cardiac arrest. French 7. 8. 9. 10. 11. 12. Ministry of Health. Ann Fr Anesth Reanim 2009; 28: 182-90. Belohlavek J, Kucera K, Jarkovsky J, Franek O, Pokorna M, Danda J, et al. Hyperinvasive approach to out-of-hospital cardiac arrest using mechanical chest compression device, prehospital intraarrest cooling, extracorporeal life support and early invasive assessment compared to standard of care. A randomized parallel groups comparative study proposal. “Prague OHCA study”. J Transl Med. 2012; 10: 163. Dumas F, Cariou A, Manzo-Silberman S, Grimaldi D, Vivien B, Rosencher J, et al. Immediate Percutaneous Coronary Intervention Is Associated With Better Survival After Out-of-Hospital Cardiac Arrest. Insights From the PROCAT (Parisian Region Out of Hospital Cardiac Arrest) Registry. Circ Cardiovasc Interv 2010; 3: 200-7. Doig CJ, Zygun DA. (Uncontrolled) donation after cardiac determination of death: a note of caution. J Law Med Ethics 2008; 36: 760-5. Bracco D, Noiseux N, Hemmerling TM. The thin line between life and death. Intensive Care Med 2007; 33: 751-4. Rodríguez-Arias D, Ortega I. Protocols for Uncontrolled donation after circulatory death. Lancet. 2012; 379: 12756. Matesanz R, Coll Torres E, Dominguez-Gil Gonzalez B, et al. [Donación en asistolia en España: situación actual y recomendaciones]. ONT. 2012. Madrid. Cite this article as: Ortega-Deballon I, De La Plaza-Horche E. A comprehensive approach to refractory cardiac arrest: saving more lives one way or another. Heart, Lung and Vessels. 2014; 6(3): 149-151. Source of Support: Nil. Disclosures: None declared. Heart, Lung and Vessels. 2014, Vol. 6 151 EXPERT OPINION Heart, Lung and Vessels. 2014; 6(3): 152-156 152 A simplified minimally invasive approach to mitral valve surgery optimal access under direct vision A. Amiri, E.M. Delmo Walter, R. Hetzer Department of Cardiothoracic and Vascular Surgery, Deutsches Herzzentrum Berlin, Berlin, Germany Heart, Lung and Vessels. 2014; 6(3): 152-156 ABSTRACT With increasing enthusiasm in minimally invasive surgery, several approaches and access are being performed with great precision. In this report, we illustrate and describe a minimal invasive approach to mitral valve surgery with optimal access under direct vision, the indications and patient selection, the surgical techniques, its advantages over the other approaches, and its simplicity and reproducibility. Keywords: mitral valve repair, minimally-invasive approach, anterolateral thoracotomy, cardiopulmonary bypass, myocardial protection. INTRODUCTION Over the past decade, minimally invasive approaches to mitral valve surgery have been commonly used at many centers around the world with excellent short- and long-term outcomes (1-5). There has been great enthusiasm about their performance because they have proven to be at least as good and safe as the standard sternotomy approach, even in elderly patients. A variety of approaches (6-8) have been reported, aimed at reducing surgical trauma and postoperative morbidity while remaining safe and effective. Some approaches have been aided by the use of thoracoscopy, specially designed retractors and surgical clamps as Corresponding author: Aref Amiri, MD Deutsches Herzzentrum Berlin Augustenburger Platz 1 13353 Berlin, Germany e-mail: amiri@dhzb.de well as special instruments for long-distance knot-tying. The right thoracotomy approach has been the most appealing, for reasons of cosmesis and reduced trauma. However minimally invasive mitral valve surgery (MIMVS) is performed, the most important consideration is that the approach must yield results equal to or better than those of the approaches it modifies or replaces. There has been increasing interest in simplifying the operation so that it can be widely applied to benefit patients. This report illustrates a simplified, reproducible minimally invasive approach with optimal access to and exposure of the mitral valve (MV) under direct vision. Patient selection We applied this approach to all patients with moderate and severe MV insufficiency and/or stenosis of various etiologies, with no concomitant coronary artery disease or aortic valve regurgitation. Even complex re- Heart, Lung and Vessels. 2014, Vol. 6 Optimal minimally invasive mitral valve surgery pair procedures for severe bileaflet prolapse in patients with Barlow’s disease can be successfully performed through this approach. MV surgery using any commercially available prostheses can be performed with the same reliability in patients in whom the mitral valve is not amenable to repair. It is also a useful alternative for patients requiring MV procedures after a previous cardiac operation, particularly in those with patent coronary artery bypasses or previous aortic valve replacement. This may also be applied in patients who had had surgeries via right thoracotomy approach. Assessment of mitral valve lesions The degree of MV insufficiency or stenosis is estimated by means of standard echocardiographic measurements. Assessment of MV function includes measurement of the mitral annulus, evaluation of leaflet mobility and coaptation, determination of mitral valve orifice area, mitral flow assessment using continuous wave Doppler, and valve anatomy evaluation as to valve thickness and pliability and morphology of the subvalvular apparatus. Standard guideline in MIMVS using the simplified approach Because the mitral valve is a posterior structure, excellent exposure can be established through the right anterolateral thoracotomy. A simplified right anterolateral thoracotomy approach includes a 10-12 cm incision, either direct aortic or peripheral arterial cannulation and direct bicaval canulation, with standard retractors. Surgical technique Under general anesthesia using a double lumen endotracheal tube, the patient is placed in left lateral position with the chest elevated to about 45-60° (Figure 1A). The right arm is placed over the head at approx- 153 Figure 1A - Position of the patient. Figure 1B - Anterolateral thoracotomy incision (10-12 cm) at the 5th intercostal space. imately 120° with the elbow joint in the right-angle position. The operating table is rotated leftwards and the patient is bent at the 12th thoracic vertebra. Transcutaneous defibrillation pads (Philips Multifunction Electrode Pads, Philips, Amsterdam, The Netherlands) are placed at the left lateral chest wall and right shoulder. A 10-12 cm skin incision over the fifth anterolateral intercostal space is made beginning in the skin fold below the right breast (Figure 1B). The right lung is deflated and the chest cavity is entered without division or resection of any bone. Standard chest retractors are used and, under direct vision, the pericardium is incised parallel and approximately 3-4 cm anterior to the phrenic nerve. Several pericardial edge retention sutures are placed anteriorly and posteriorly. This pro- Heart, Lung and Vessels. 2014, Vol. 6 A. Amiri, et al. Figure 1C - Optimal exposure of intracardiac structures with aortic and bicaval cannulation within the same incision. 154 vides optimal and excellent visualization and access to the ascending aorta and superior vena cava. Heparin is administered and arterial cannulation is performed either directly in the ascending aorta or in the common femoral artery (only in patients with a small and narrow chest) with direct bicaval cannulation with caval snares (Figure 1C). Adequate myocardial protection is achieved with intermittent blood cardioplegia through a cardioplegia needle in the aortic root. The ease and handling of myocardial protection provides no difficulty and is comparable when the approach is through a standard median sternotomy. After clamping the ascending aorta with a straight clamp having a flexible handle, the left atrium is opened by incising the interatrial groove with a vent cardiotomy sucker placed directly towards the left pulmonary veins. A retractor is placed to elevate the interatrial septum, exposing the mitral valve, and the valve is meticulously inspected to determine the precise nature of the lesion. Leaflet coaptation is assessed with transvalvular saline injection under pressure. Using a nerve hook, leaflet coaptation and the presence of sufficient tissues along the coaptation plane are evaluated. Depending on the results of this evaluation, mitral valve repair or replacement is performed under direct vision, using precisely the same approach as in a conventional median sternotomy. Mitral valve repair Modified Gerbode plication plasty (9) is applied for posterior leaflet prolapse, ruptured chordae and in ischemic mitral insufficiency (MI). Prolapse can occur anywhere along the posterior leaflet but is most commonly found in the region of P2, which may lead to chordal rupture. In this technique, the flail segment is plicated towards the left ventricle in a Vshaped fashion with interrupted mattress sutures using double-ended 3-0 polypropylene with untreated autologous pericardial pledgets. Hence, the P1 segment is attached to the P3 segment. When competence and size are satisfactory a strip of untreated autologous pericardium is sutured continuously onto the posterior annulus without further annular narrowing. Heart, Lung and Vessels. 2014, Vol. 6 Optimal minimally invasive mitral valve surgery Modified Paneth-Hetzer posterior annulus shortening technique (9) is utilized for severe annular dilatation and ischemic MI. This is performed by running a pericardialpledgeted 3-0 polypropylene suture through the fibrous body of the trigone and tying it. Then it is run along the annulus from one trigone towards the middle of the posterior annulus. The same is done on the opposite trigone. These sutures are then tied over an appropriately sized Ziemer-Hetzer valve sizer to prevent over-narrowing of the valve orifice. The valve is then tested with saline injection for competence. Using the same needles, both sutures are passed onto an autologous pericardial strip. Then, with a continuous suture, the pericardial strip is attached to the posterior annulus from the midsegment towards the trigone. The leaflet coaptation is tested by a forceful injection of saline through the valve, to look for residual regurgitation. We also use this technique in anterior leaflet prolapse; the then wider coaptation plane will eliminate prolapse. Evaluation of the adequacy of repair After MV repair, it is obligatory to assess the valve function before closure of the atrium and separation from cardiopulmonary bypass (CPB). This is done by transvalvular saline injection with a bulb syringe under pressure. Any remaining areas contributing to significant incompetence must be attended to before closure of the atrium. Once de-airing has been completed and extracorporeal circulation is discontinued, the repair result must be further evaluated with intraoperative transesophageal echocardiography (TEE) in order to test for inadequate mitral opening area, residual incompetence, myocardial ischemia due to coronary kinking and presence of the systolic anterior motion (SAM) phenomenon. Immediate and prompt correction must be made if the repair is shown to be unsatisfactory. Regardless of the underlying pathology and techniques used, no patient should be discharged from the operating room with more than minimal MI. Mitral valve replacement If it is established that the mitral valve lesion is not amenable to repair, the valve is replaced, with either a mechanical or biological prosthesis, in accordance with the patient’s wishes. In both procedures, no specially designed instruments are required, and the knots may be tied by hand or with a knot pusher. Several strategies of knot tying have been learned with experience, such as having the assistant hold up the annular sutures during knot tying. After completion of the procedure, the left ventricle is vented with a catheter positioned across the valve and the atriotomy is closed. Concomitant Maze procedure with radiofrequency ablation may also be performed, when necessary. Caval snares are snugged tight when tricuspid valve reconstruction (double-orifice-valve technique) (10) or closure of patent foramen ovale are performed, and these procedures are done through right atriotomy. Throughout the procedure, a vacuum-assisted venous drain in the heart-lung machine is used and carbon dioxide is infused into the operative field to decrease the chance of air embolism. Complete evacuation of intracardiac air is performed through the aortic root and left atrium and confirmed by TEE. Temporary atrial and ventricular pacing wires are placed before releasing the aortic clamp. Defibrillation, when necessary, is administered through the external defibrillator pads. Once the hemodynamic status is stable, cardiopulmonary bypass is discontinued and Heart, Lung and Vessels. 2014, Vol. 6 155 A. Amiri, et al. 156 decannulation is performed. Transesophageal echocardiography is mandatory at the moment to document the repair results or prosthetic function. The right pleural space and mediastinum are drained through the 7th intercostal space with two chest tubes and the intercostal spaces are closed with five or six pericostal sutures. associated with balloon malposition or migration (11). This simplified approach combines good cosmesis with optimal exposure of the mitral valve and all the cardiac structures, is readily applicable and is not associated with a steep learning curve because one employs conventional cannulation and clamping techniques. REFERENCES CONCLUSION This simplified minimally invasive approach to mitral valve surgery with optimal access to all cardiac structures and optimal exposure of the mitral valve under direct vision offers distinct advantages, including direct aortic root and caval cannulation performed with ease and without obscuring the operative field, optimal exposure and overview of the operative field, controlled myocardial protection, and adequate de-airing. Although the skin incision is 5-8 cm longer than in the conventional minimally invasive technique, besides providing good cosmesis and an acceptable postoperative scar, it outweighs the placement of additional skin incisions for the other cannulae or the aortic clamp, and obviously avoids the potential complications related to femoral vessel cannulation. Additionally, standard aortic cross-clamping and antegrade cardioplegia delivery obviate the need for specialized endovascular occlusive balloons, hence avoiding the potential complications 1. Davierwala PM, Seeburger J, Pfannmueller B, Garbade J, Misfeld M, Borger MA, et al. Minimally invasive mitral valve surgery: “The Leipzig experience”. Ann Cardiothorac Surg. 2013; 2: 744-50. 2. Galloway AC, Schwartz CF, Ribakove GH, Crooke GA, Gogoladze G, Ursomanno P, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg. 2009; 88: 1180-4. 3. Misfeld M, Borger M, Byrne JG, Chitwood WR, Cohn L, Galloway A, et al. Cross-sectional survey on minimally invasive mitral valve surgery. Ann Cardiothorac Surg. 2013; 2: 733-8. 4. Cohn LH, Byrne JG. Minimally invasive mitral valve surgery: current status.Tex Heart Inst J. 2013; 40: 575-6. 5. Rittwick B, Chaudhuri K, Crouch G, Edwards JR, Worthington M, Stuklis RG. Minimally invasive mitral valve procedures: The current state. Minim Invasive Surg. 2013; 2013: 679276. 6. Angouras DC, Michler RE. An alternative surgical approach to facilitate minimally invasive mitral valve surgery. Ann Thorac Surg. 2002; 73: 673-4. 7. Tam RK, Ho C, Almeida AA. Minimally invasive mitral valve surgery. J Thorac Cardiovasc Surg. 1998; 115: 246-7. 8. Modi P, Chitwood WR Jr. Retrograde femoral arterial perfusion and stroke risk during minimally invasive mitral valve surgery: is there cause for concern? Ann Cardiothorac Surg. 2013; 2: E1. 9. Hetzer R, Delmo Walter EM. No ring at all in mitral valve repair: indications, techniques and long-term outcome. Eur J Cardiothorac Surg. 2014; 45: 341-51. 10. Hetzer R, Komoda T, Delmo Walter EM. How to do the double orifice valve technique to treat tricuspid valve incompetence. Eur J Cardiothorac Surg. 2013; 43: 641-2. 11. Grocott HP, Smith MS, Glower DD, Clements FM. Endovascular aortic balloon clamp malposition during minimally invasive cardiac surgery: detection by trranscranial Doppler monitoring. Anesthesiology. 1998; 88: 1396-9. Cite this article as: Amiri A, Delmo Walter EM, Hetzer R. A simplified minimally invasive approach to mitral valve surgery optimal access under direct vision. Heart, Lung and Vessels. 2014; 6(3): 152-156. Source of Support: Nil. Disclosures: None declared. Acknowledgment: We thank Anne Gale for editorial assistance. Heart, Lung and Vessels. 2014, Vol. 6 REVIEW ARTICLE Heart, Lung and Vessels. 2014; 6(3): 157-170 Acute right heart syndrome in the critically ill patient V. Zochios, N. Jones Cardiothoracic Intensive Care Unit, Papworth Hospital NHS Foundation Trust, Papworth Everard, Cambridge, UK Heart, Lung and Vessels. 2014; 6(3): 157-170 ABSTRACT Acute right heart syndrome is a sudden deterioration in right ventricular performance, resulting in right ventricular failure and confers significant in-hospital morbidity and mortality. In critically ill patients, the syndrome is often undiagnosed and untreated, as these patients do not usually exhibit the common clinical manifestations of the condition, making the diagnosis challenging for the intensivist. In this narrative review we focus on the pathophysiology of acute right heart syndrome, in critical illness, diagnostic modalities used to assess right ventricular function and management of acute right heart syndrome, including mechanical ventilation strategies and circulatory support. Keywords: critical illness, right heart failure, pulmonary artery pressure, mechanical ventilation, echocardiography. INTRODUCTION Acute right heart syndrome (ARHS) may be defined as sudden deterioration in the right ventricular (RV) function and failure of the RV of the heart to deliver adequate blood flow to the pulmonary circulation, resulting in systemic hypoperfusion (1). In the context of critical illness, ARHS is associated with poor outcomes and increased mortality (2). Evidence of central venous pressure (CVP) overload in conjunction with RV contractile dysfunction, is usually present in ARHS (1). We searched PubMed, EMBASE, Cochrane library and Google Scholar, for articles reCorresponding author: Dr Vasileios Zochios Cardiac Critical Care Fellow Department of Cardiothoracic Anesthesia and Intensive Care Cardiothoracic ICU Papworth Hospital NHS Foundation Trust Papworth Everard, Cambridge, UK, CB23 3RE e-mail: vasileioszochios@doctors.org.uk porting on RV dysfunction and failure. The relevant papers were extracted in full and references from extracted papers were checked for any additional relevant articles. An overview of the ARHS pathophysiology, diagnostic tools for the assessment of the acutely failing RV in critical illness and measures including vasoactive agents, ventilatory strategies and mechanical support is provided in the current paper. THE RV IN HEALTH The main functions of the RV are: a) maintenance of adequate pulmonary perfusion pressure in order to deliver desaturated mixed venous blood to the respiratory membrane; b) maintenance of low systemic venous pressure in order to prevent organ congestion. Heart, Lung and Vessels. 2014, Vol. 6 157 V. Zochios, et al. 158 The RV is anatomically adapted for the generation of low-pressure perfusion and it is very sensitive to changes in afterload. (3). The differences between the structure and function of the RV compared to the left ventricle (LV) are outlined in Table 1 and Figure 1 compares the pressure-volume (PV) loop of the RV with that of the LV (3-5). THE RV IN CRITICAL ILLNESS ARHS is not necessarily associated with an increase in pulmonary vascular resistance (PVR) and pulmonary arterial hypertension (PAH) (6). The syndrome can be due to RV pressure/volume overload or RV contractile dysfunction (1). Consequence is low cardiac output (CO) with low mean arterial pressure (MAP), exacerbating RV dysfunction. Thus, “RV failure begets RV failure” leading to a progressive downward spiral of worsening ischemia, myocardial dysfunction and shock. In mechanically ventilated patients with ARHS, low CO is multifacto- rial and could be due to RV systolic dysfunction, tricuspid regurgitation, ventricular interdependence (dilatation of the RV shifting the interventricular septum toward the left and decreasing the LV distensibility and preload), arrhythmias or suboptimal preload (6). RV diastolic dysfunction causes impaired RV filling and high diastolic RV and right atrial (RA) pressures leading to organ congestion (6). The causes and precipitating events of ARHS are summarized in Table 2 (7-15). ARHS in Acute Respiratory Distress Syndrome (ARDS) ARDS is one of the most common causes of ARHS secondary to RV pressure overload (acute cor pulmonale). In critically ill ventilated patients with ARDS, ARHS occurs in 61% of patients submitted to conventional tidal volume mechanical ventilation (MV) and 25% of those receiving lung protective MV using low tidal volumes (15, 16). Apart from MV, the pathologic features of the syndrome per se, contribute to increased pulmonary vascular tone, acute Table 1 - Differences between RV and LV (3-9, 25). Structure Shape in cross section Enddiastolic wall thickness Enddiastolic volume Systolic Pressure Ejection fraction Coronary perfusion Response to disease Pressurevolume loop RV Inflow region, infundibulum Semicircular /serpentine shape ≤3 mm 49-101 ml/m2 25 mmHg 40-68% Continuous (systole & diastole) Better adaptation to volume overload states, higher compliance than LV Trapezoidal with poorly defined isovolumetric periods - RV pressure rise and ejection continue despite fall in RV pressure. LV No infundibulum mitro-aortic continuity Circular ≤11 mm 44-89 ml/m2 120 mmHg 57-74% Almost exclusively in diastole Better adaptation to pressure overload states Rectangular - rapid rise and fall of pressure with ejection RV = right ventricle; LV = left ventricle. Heart, Lung and Vessels. 2014, Vol. 6 Acute right heart syndrome in critical illness 159 Figure 1 - Pressure-volume (P-V) loops for RV and LV. Once RV pressure reaches the PA pressure, the pulmonary valve opens. Little time is spent in isovolumetric contraction, giving a triangular-shaped RV P-V loop, in contrast to the almost square loop of the LV (25). (Adopted from: Kevin LG, Barnard M. Right ventricular failure. Contin Educ Anaesth Crit Care Pain 2007; 7: 89-94). Permission to reproduce granted under Oxford university press’s general terms. RV = right ventricle; LV = left ventricle. Table 2 - Precipitating events / causes of ARHS in the ICU (7-15). RV pressure overload (endothelial dysfunction, vasoconstriction, mechanical obstruction) 1. Massive pulmonary embolism. 2. Acute Respiratory Distress Syndrome. 3. Deteriorating chronic pulmonary arterial hypertension. 4. Post cardiothoracic surgery 5. Mechanical ventilation. 6. Pulmonary valve stenosis. 7. Hypoventilation state RV volume overload 1. Pulmonary or triscupid valve regurgitation. 2. Left to right shunt due to inter-atrial defect. 3. Anomalous pulmonary venous return. 4. Hyperthyroidsm RV contractile dysfunction 1. RV myocardial infarction (via negative inotropic effect or arrhythmia). 2. Relative RV ischemia secondary to RV pressure or volume overload. 3. Intrinsic myocardial disease e.g RV cardiomyopathy, sepsis (cytokine induced myocardial depression), inflammatory effects of cardiopulmonary bypass, myocarditis. 4. Pericardial disease e.g constrictive pericarditis, tamponade (causing impaired diastolic filling). 5. Left ventricular assist device (due to acute unloading of the LV). LV dysfunction (by increasing pulmonary venous and pulmonary arterial pressure, myocardial ischemia, LV dilatation leading to restricted RV diastolic function). ARHS = acute right heart syndrome; ICU = intensive care unit; RV = right ventricular; LV = left ventricular. Heart, Lung and Vessels. 2014, Vol. 6 V. Zochios, et al. 160 pulmonary arterial hypertension (PAH) and cor pulmonale. Contributors to elevated pulmonary vascular resistance (PVR) in ARDS include: vasoconstrictor: vasodilator imbalance (excess ET-1, 5HT, PDE, reduced NO and prostanoids), endothelial injury, hypoxic pulmonary vasoconstriction (80% arteriolar), hypercapnia (including permissive hypercapnia), acidemia, in situ thrombosis and pulmonary vascular remodelling (muscularization of non-muscularized arteries) (8, 16, 17). tered vaso-reactivity, despite concomitant decrease in systemic vascular resistance (SVR). Substantial increases in PVR also occur when the left ventricle needs to considerably increase the cardiac output in order to compensate for the fall in the SVR, causing further increase in RV afterload (21, 22). ARHS in the setting of massive pulmonary embolism (PE) Critically ill patients are at risk of PE despite thromboprophylaxis (3, 18). In a tenyear retrospective study, Vieillard-Baron et al. (19) showed that ARHS was present in 61% of medical intensive care unit (ICU) patients with massive PE and carried a 23% mortality. The normal RV can generate a mean pulmonary artery pressure up to 40 mmHg, requiring 50-75% of the pulmonary vasculature to be occluded by emboli before acute RV failure occurs (19). Hypoxemia induced by the emboli results in pulmonary vasoconstriction and the physiological response to platelet activation leading to release of vasoactive agents such as serotonin, thromboxane and histamine, causes further increase in PVR and RV pressure overload (19, 20). CLINICAL FEATURES The clinical features of ARHS, including acute onset shortness of breath, orthopnea and bilateral lower extremity edema, are non-specific and difficult to identify in the sedated critically ill patient (6). Increased oxygen requirements or sudden cardiovascular collapse might be the chief clinical manifestations of ARHS in a mechanically ventilated patient (23). Other prominent clinical signs include atrial or ventricular arrhythmias, raised jugular venous pressure and gallop rhythm at the left sternal edge, systolic murmur of tricuspid regurgitation, organomegaly, signs of deep venous thrombosis (in the context of venous thromboembolism) (6). It is important to consider ARHS in persistent respiratory weaning failure (RV dysfunction leads to an imbalance between ventilator needs and cardiorespiratory capacity), especially in patients with LV systolic dysfunction (6, 9, 24, 25). A high index of suspicion is needed in high risk patients such as those with pre-existing PAH and recent deep venous thrombosis (25). ARHS in sepsis In severe sepsis and septic shock the RV function might be impaired. RV systolic dysfunction in sepsis is directly associated with markers of endothelial dysfunction (endothelin 1, vascular cellular adhesion molecule 1) and directly related to the severity of sepsis (21). A proposed mechanism for ARHS in sepsis is increased PVR secondary to sepsisinduced endothelial cell injury and al- DIAGNOSIS OF ARHS IN THE CRITICALLY ILL BEDSIDE STUDIES Available bedside studies include: chest X-ray (CXR), electrocardiography (EKG), arterial blood gas (ABG) analysis, hemodynamic and echocardiographic diagnostic tools. Heart, Lung and Vessels. 2014, Vol. 6 Acute right heart syndrome in critical illness Chest X-ray Enlargement of the main pulmonary artery and regional oligemia are seen in massive PE. However, CXR cannot be utilized to confirm the diagnosis of ARHS and should only contribute to the diagnostic approach by ruling out conditions that mimic ARHS in the ICU, such as atelectasis, pleural effusions, pulmonary edema and pneumothorax (6). Electrocardiography Kucher et al. showed that Qr in V1 is a strong predictor of RV dysfunction, and it is highly associated with troponin leakage and myocardial shear stress (26). It has also been demonstrated that in patients with right bundle branch block, R duration in lead V1>100 ms is predictive of RV systolic dysfunction (43). Other EKG findings suggestive of RV strain include inversion of T waves in leads V1V4 and the classic S1Q3T3 pattern. Acute anterior Q-wave pattern in leads V1-V3, as well as a right-sided Q pattern in leads V3R–V6R, might suggest RV infarction (12). EKG, although specific, lacks sensitivity (11). Arterial blood gas analysis ABG analysis may reveal grossly impaired gas exchange and low cardiac output might result in acidemia with lactic acidosis due to tissue hypoperfusion (27). Hemodynamic bedside diagnostic modalities Central venous catheters and central venous pressure An accurately placed central venous catheter (in the superior vena cava), can provide information on CVP and used as a surrogate for RV end-diastolic volume (RVEDV) and RV end-diastolic pressure (RVEDP) (25, 28). In severe tricuspid regurgitation causing ARHS, a broad, tall systolic c-v wave is seen due to abnormal systolic fill- ing of the right atrium (RA) and the CVP trace is said to be ventricularized because it resembles right ventricular pressure (25, 28). RVEDP reflects RVEDV (which is proportional to preload) only when ventricular compliance is normal. Therefore, in conditions such as PAH, tamponade and myocardial ischemia, where RV compliance is decreased, CVP is likely to be raised and cannot be accurately assessed (28, 29). Right heart catheterization Right heart catheterization using a pulmonary artery catheter (PAC) is frequently required when ARHS is clinically suspected and interpretation of imaging studies is difficult or inconclusive. Hemodynamic data obtained from an accurately placed PAC, by thermodilution, may provide diagnostic clues and guide therapy. PAC allows direct simultaneous measurement of RA, RV, PA and pulmonary artery wedge pressures and indirect measurement of cardiac output, cardiac index (CI), RV stroke work index, mixed venous oxygen saturation, PVR and SVR (29, 30). Hemodynamically, ARHS is suspected if RA pressure >8-10 mmHg, or RA pressure to pulmonary capillary wedge pressure ≥0.8 (isolated RV failure) and CI is low. In the presence of RV-PA gradient >25 mmHg, RV outflow tract obstruction should be excluded by echocardiography (31). In the context of PAH and suspected ARHS, right heart catheterization allows assessment of left-sided heart disease and its contribution to PAH. Besides, calculation of PVR and SVR help decide whether pulmonary or systemic vasodilators/pressors are needed and monitor response to therapy (29). In patients with pre-existing PAH, a decrease in PA pressure might reflect low RV ejection fraction and worsening RV dysfunction (12). Heart, Lung and Vessels. 2014, Vol. 6 161 V. Zochios, et al. 162 Arterial pulse contour analysis Arterial pulse contour analysis enables calculation of CO, pulse pressure variation (PPV), stroke volume variation (SVV) and SVR, from the arterial pulse pressure waveform, in mechanically ventilated patients. Dynamic indices (SVV, PPV) have been used to predict preload responsiveness and monitor the hemodynamic effect of volume expansion in critically ill patients (32). Wyler Von Ballmoos et al. reported that PPV is not accurate predictor of fluid responsiveness in mechanically ventilated patients with acute PAH (at risk of ARHS), early after cardiac surgery and in septic shock (33). In the context of a pressure overloaded RV, increased PPV values are related to an increase in the RV afterload and not to a decrease in RV preload and therefore, further volume expansion could potentially be harmful (33, 34). However, it could be reasonably contended that lack of response to a fluid challenge, while PPV or SVV is high, could be seen as an indicator of RV dysfunction necessitating further investigations (35). Bedside imaging modalities Echocardiography Transthoracic (TTE) and transesophageal echocardiography (TEE) are bedside diagnostic tools which also provide rapid risk stratification and could potentially direct therapeutic strategies. In experienced hands, echocardiography allows assessment of the RV performance and loading conditions. Useful echocardiographyderived measures of RV function, when ARHS is clinically suspected are outlined in Table 3 (36). TTE is an easy and non-invasive way to assess the size and kinetics of the RV. The diagnosis of ARHS due to RV pressure overload, with TTE, has good positive predictive value for indirect diagnosis of massive PE (37). Main limitations of TTE in critically ill patients ventilated with high level of positive end-expiratory pressure (PEEP) include: inadequate imaging due to interposition of the inflated lung between the heart and the chest wall, low diagnostic accuracy in the patients with pre-existing cardiopulmonary disease, the operator dependent nature of TTE (37). Table 3 - Echocardiographic quantitative parameters pointing towards ARHS (36-38). RV systolic dysfunction 1. TAPSE <16 mm 2. 2D RV FAC < 35% 3. RIMP >0.4 by pulsed Doppler and >0.55 by tissue Doppler RV diastolic dysfunction 1. E/A <0.8 by tissue Doppler 2. E/A >2.1 by tissue Doppler Dilated RV chamber 1. Diameter >32 mm at the base 2. Diameter >35 mm at the mid-level 3. Longitudinal dimension >86 mm RVOT dilatation 1. Diameter >27 mm at end-diastole at the level of pulmonary valve RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; 2DRVFAC = two-dimensional right ventricular fractional area change; RIMP = right ventricular index of myocardial performance; E/A = early (E) to late (A) ventricular filling velocities ratio; RVOT = right ventricular outflow tract . Heart, Lung and Vessels. 2014, Vol. 6 Acute right heart syndrome in critical illness RV function, size and shape, are more accurately assessed with TEE. It has been suggested that in the presence of significant and otherwise unexplained RV strain without clots present on TTE, TEE should rapidly follow at the bedside, providing there is local availability and expertise (38). TEE is a semi-invasive procedure and commonly reported complications associated with TEE in critically ill patients receiving MV, include: hypo- or hypertension, dysrrhythmias, trauma to the gastrointestinal tract, hypoxemia and dislodgment of endotracheal or nasogastric tubes. The over-all complication rate associated with TEE use is low and it is estimated to be approximately 2.6% (38). Additional imaging modalities Computed tomography (CT) CT pulmonary angiography (CTPA) is being used increasingly as a diagnostic tool in PE, with documented sensitivities of 50-100% and specificities of 81-100% (39). CTPA has become the preferred diagnostic modality for suspected ARHS due to PE, in hemodynamically stable ICU patients (66). Chest CT signs suggestive of ARHS include: flattening or displacement of the intraventricular septum toward the LV, reflux of contrast into the inferior vena cava, RV diameter (RVD) to LV diameter (LVD) ratio on axial sections greater than 1.0 (RVDaxial/LVDaxial >1) (39). Cardiovascular Magnetic Resonance (CMR) CMR is the most sensitive method to assess the RV size and function. Imaging quality is not influenced by acoustic windows or pre-existing cardiopulmonary disease (40). However, CMR is rarely used for ICU patients receiving MV, as the MR environment carries significant risks to patients during transportation and prolonged periods in the MR scanner. LABORATORY TESTS The usefulness of laboratory tests such as D-dimmer, troponins and B-type natriuretic peptide levels, as diagnostic tests in ICU patients with suspected RV failure, is limited, as they are non-specific and confounded in the context of critical illness (41, 42). In summary, in critically ill patients with clinically suspected ARHS, echocardiography (TTE and/or TEE) and right heart catheterization are the preferred diagnostic modalities. If PE is the most likely cause of ARHS, then CTPA is necessary to confirm the diagnosis, provided the patient is suitable for transfer to radiology. In any given scenario, the diagnostic approach will depend upon the expertise and availability of the different diagnostic modalities. TREATMENT The principles and key components of ARHS management include reversal of precipitating events and control of contributing factors (hypoxemia, hypercapnia, anemia, acidemia, sepsis, dysrrhythmias), fluid volume optimization, maintenance of perfusion pressure, positive inotropy, use of pulmonary vasodilators and protective MV (12, 43). The management principles and strategies will depend upon the primary hemodynamic pathology. Control of contributing factors, general ICU care and reversible causes of ARHS Infection prevention, treatment of sepsis in accordance with the surviving sepsis campaign bundles, normoxia, normocapnia, thromboprophylaxis, peptic ulcer prophylaxis, correction of acid-base imbalance and electrolytes and glycemic control are mandatory and applied to most ICU patients (12). Pulmonary vasodilators and/ Heart, Lung and Vessels. 2014, Vol. 6 163 V. Zochios, et al. 164 or inodilators (in acutely decompensated PAH), thrombolysis (in massive PE), revascularization (in RV infarction) and sequential AV pacing and/or cardioversion (in significant dysrrhythmias), could potentially correct the abnormal RV physiology (44). Optimization of intravascular fluid status Fluid loading in ARHS remains controversial. RV ejection fraction is dependent on RV pre-load, in the abscence of PAH and it is likely that RV output will be inadequate in hypovolemia. The RV can increase the stroke work through an increase in RV free wall stretch (via the Frank-Starling mechanism) (29). Therefore, optimization of preload may improve RV ejection fraction. The role of CVP as a guide to fluid therapy remains controversial. A systematic review of 24 studies demonstrated a poor relationship between CVP and intravascular fluid status and the inability of CVP/delta-CVP to predict the hemodynamic response to a fluid challenge (32, 45). Depending on where the patient is on the Frank-Starling curve, some may be adequately resuscitated with a CVP of 6-7 mm Hg, while others may still be intravascularly volume depleted at a CVP of 10 mm Hg (31). In a recent meta-analysis, Marik et al. showed that there is paucity of data to support the widespread practice of using CVP to assess intravascular fluid status and guide fluid therapy (46). More reliable hemodynamic assessment tools, such as PAC, pulse contour analysis, TTE and/ or TEE when available, may be utilized to guide fluid therapy in ARHS (32). Mercat et al. showed that in critically ill medical patients with circulatory failure (defined by CI <2.5 L/min/m2), due to massive PE, fluid loading with 500 ml of colloid increased the cardiac index significantly and improved hemodynamic status (47). If the hemodynamic response to ini- tial fluid challenge is poor, in the context of ARHS, further volume loading may cause RV overdistension, increased ventricular interdependence, decreased LV filling and RV ischemia, leading to worsening shock (44). In RV volume overload, acute kidney injury due to venous congestion (cardiorenal syndrome), continuous veno-venous hemofiltration (CVVH) facilitates greater clinical improvement compared with aggressive diuretic therapy, in heart failure patients, who are diuretic resistant (48). The role of vasopressors in ARHS In order to preserve adequate right coronary blood flow, systemic pressure should be maintained above the PA pressures. It has been shown that in patients with sepsis, PAH and RV dysfunction, norepinephrine increases systemic pressure through alpha-1 receptor agonism and may improve the RV oxygen supply/demand ratio, but this potentially beneficial effect on RV ejection fraction may be offset by a concomitant increase in PVR and RV afterload, at high doses (>0.5 mcg/kg/min) (43, 49). Besides, norepinephrine, through beta-1 receptor agonism could potentially improve RV-PA coupling and CO (22). Low dose vasopressin (0.033-0.067 U/min) mediates pulmonary arterial vasodilation and may be useful in vasodilatory shock and pulmonary vascular dysfunction, especially in norepinephrine resistant patients (43, 49, 50). Inotropes and inodilators in ARHS Dobutamine (beta-1 receptor agonist) can be used as the first-line inotropic agent in ARHS due to RV contractile dysfunction. Low dose dobutamine (2-5 mcg/kg/min) increases CI, SV and decreases PVR and SVR (12, 43, 51). At higher doses (>10 mcg/ kg/min) dobutamine causes tachycardia, increased oxygen consumption, increased PVR and leads to systemic hypotension Heart, Lung and Vessels. 2014, Vol. 6 Acute right heart syndrome in critical illness and addition of a vasopressor might be required (12, 51). High quality evidence suggests that dopamine is associated with increased tachyarrhythmias and is not recommended in cardiogenic shock (45). It has been demonstrated that in patients with septic shock and ARHS, who are unresponsive to fluid loading, dopamine or dobutamine, epinephrine improves RV contractility in spite of a rise in mean PAP by 11% (p<0.05) (52). Selective phosphodiresterase (PDE) III inhibitors (enoximone, milrinone, amrinone), augment myocardial contractility and cause systemic and pulmonary vasodilaton, by increasing cyclic adenosine monophosphate (cAMP) and thus reducing PA pressures and improving RV function in patients with ARHS due to pressure overloaded RV (43). Systemic hypotension should be expected and addition of a vasopressor might be needed. It has been demonstrated that levosimendan, a calcium sensitizer with pulmonary vasodilator properties (inodilator), improves RV performance in ARHS secondary to sepsis-induced ARDS and in experimental ARHS restores RV-PA coupling better than dobutamine (53). In ARHS, levosimendan has been shown to reduce the increased RV afterload and ventricular interdependence, improve RV contractility and RV diastolic function, without significant increase in oxygen consumption, mediated by opening of sarcolemmal and mitochondrial potassium-adenosine triphosphate channels (54, 55). Although levosimendan is indicated for the treatment of acute heart failure (class of recommendation IIa, level of evidence B), it is has not yet been approved in all countries (54, 55). Pulmonary vasodilation in ARHS The goals of pulmonary vasodilation in ARHS are: 1) decrease PVR and impedance; 2) increase RV stroke volume and output; 3) avoid systemic hypotension and maintain coronary perfusion; 4) avoid hypoxemia from ventilation-perfusion mismatch. It is recommended that inhaled nitric oxide (NO), which increases intra-cellular cyclic guanosine monophosphate (cGMP), should be considered as short term therapy to improve PaO2/FiO2 ratio and CO, in ventilated patients with ARHS secondary to ARDS (43). It has also been suggested that NO may be effective in stabilizing patients with ARHS due to massive PE until more definitive treatment is available (29, 56). Prostanoid formulations (epoprostenol, iloprost) are potent pulmonary and systemic vasodilators with anti-thrombotic and anti-proliferative actions. They reduce PVR and improve RV function and they have been used in ARHS due to RV pressure overload (57). It has been shown that use of intravenous epoprostenol in mechanically ventilated patients with ARDS reduces PVR and improves RV performance (58). Sildenafil, a PDE 5 inhibitor, increases downstream cGMP signaling and potentiates the beneficial effects of NO. It reduces PVR and increases CO and myocardial perfusion (29). Karakitsos et al. showed that mechanically ventilated patients with ARHS from PAH, who were dependent on dobutamine, were treated with oral sildenafil and in many cases they were successfully weaned from inotropic and ventilatory support (59). Mechanical ventilation strategies in ARHS Optimal MV ventilation management in ARHS consists of: avoidance of hypoxemia, hypercapnia, high levels of PEEP (>10 cmH2O) and both high and low extremes of lung volumes and use lung protective ventilation strategies if possible (43, 60-62). Heart, Lung and Vessels. 2014, Vol. 6 165 V. Zochios, et al. 166 The RV afterload is governed by PVR which is directly affected by changes in lung volume (61). Increased PVR occurs at both low and high lung volumes. At low volumes this is due to the elastic recoil forces of the lung parenchyma leading to extra-alveolar vessel collapse and terminal airway collapse leading to alveolar hypoxia and hypoxic pulmonary vasoconstriction (HPV) and at high lung volumes due to collapse of the alveolar vessels via stretch of the alveolar wall. When PVR is plotted against lung volume, a typical U-shaped curve occurs with the lowest PVR occurring at functional residual capacity (FRC) (Figure 2) (62). Schmitt et al. assessed the impact of PEEP on the RV outflow impedance using doppler data obtained by TEE, in mechanically ventilated ICU patients with ARDS. They demonstrated that high PEEP (13±4 cmH2O) was associated with increased RV afterload and worsening RV systolic dysfunction (60). The significant decrease in the incidence of ARHS (from 61% to 25%) in ARDS since ARDSnet trial was published, reflects a change in MV practice and suggests that lung protective strategies (tidal volume: 6-8 ml/kg predicted body weight (PBW), low plateau pressures and PEEP) reduce the incidence of ARHS (63). In patients with ARHS, during lung protective ventilation, permissive hypercapnia should be avoided as acute hypercapnia could lead to pulmonary vasoconstriction or exacerbate hypoxic pulmonary vasoconstriction and could potentially worsen RV dysfunction (64). A prospective observational study, which evaluated the relative roles of acute permissive hypercapnia and PEEP variations on RV function, in severe ARDS patients, showed that increasing PEEP at constant Pplat induces acute hypercapnia that may impair RV function and decrease CI. It is therefore recommended that in cases of ARHS, lung protective ventilation should be gradually adapted to limit hypercapnia and RV overload (65). In mechanically ventilated ICU patients with ARHS, refractory hypoxemia and/ or hypercapnia and high PEEP requirements, extracorporeal membrane oxygenFigure 2 - Effect of changing lung volume on pulmonary vascular resistance (PVR) (62). (Adopted from: Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child 1999; 80: 475-480). Permission to reproduce granted under BMJ Publishing Group Ltd’s general terms. RV = residual volume; FRC = functional residual capacity; TLC = total lung capacity. Heart, Lung and Vessels. 2014, Vol. 6 Acute right heart syndrome in critical illness ation (ECMO) could be used as a bridge to the recovery of respiratory function. Oxygenation and carbon dioxide clearance are provided by the extracorporeal circuit, minimizing pulmonary vasoconstriction due to hypoxemia and/or hypercapnia (66, 67). In patients with ARHS due to severe ARDS, where lung protective ventilation may not be adequate in managing hypercapnic acidosis, extracorporeal carbon dioxide (ECCO2) removal devices are an option, as they are less invasive than ECMO and may play a role in instituting “ultraprotective” lung ventilation (tidal volume: 4 ml/kg PBW) (68). It should be noted that the cardiac consequences of weaning from MV may be responsible for weaning failure in patients with ARHS. In these patients, an increase in weaning-induced RV afterload may occur due to marked increase in work of breathing, hypoxemia or high intrinsic PEEP, leading to further worsening RV enlargement during weaning. This may result in leftward shift of the interventricular septum, impeding LV diastolic filling and LV output (ventricular interdependence), causing pulmonary edema and failure to wean from MV (69). shock, after systemic thrombolysis. It can be used as a means of unloading the RV and supporting systemic circulation, in medically refractory RV failure with accompanying hypotension and end-organ failure and as a bridge to transplant (74, 75). Right ventricular assist devices (RVADs) in ARHS may be used as a bridge to recovery or transplant, or as a definitive surgical treatment, in primary RV dysfunction. In patients who are successfully weaned from the RVAD, residual RV dysfunction is compatible with survival (76). It has been suggested that RVADs should be avoided in patients with ARHS secondary to RV afterload resistance (with severely elevated PVR), as pumping blood into the PA could potentially cause worsening PAH and lung injury, whereas CO and CI remain low. In such cases VA ECMO might be more effective in off-loading the RV (77). The use of mechanical cardiovascular support devices depends largely on local availability of specialized facilities, cardiopulmonary pathophysiology expertise and operator experience. Mechanical circulatory support Low cardiac output syndrome caused by ARHS after cardiac surgery, particularly coronary artery bypass graft surgery and heart transplant, may be an indication for intra-aortic balloon pump (IABP) (70, 71). It has been demonstrated that IABP improves hemodynamics and RV efficiency in acute ischemic RV failure (72). However, a recent RCT failed to demonstrate any mortality benefit in patients with cardiogenic shock complicating acute myocardial infarction (73). Veno-arterial (VA) ECMO has been used as a salvage therapy in cases of ARHS due to massive PE and refractory cardiogenic ARHS can occur in many critical illnesses and carries substantial morbidity and mortality. ARHS is difficult to diagnose in the critically ill as those patients have ongoing physiological derangement presenting the intensive care specialists with a diagnostic dilemma. Cardiac echo and right heart catheterization are invaluable diagnostic tools in the assessment of the RV at the bedside, which also provide a rapid risk stratification and could direct treatment strategies. Characterizing, identifying and correcting reversible factors is of paramount importance. Minimizing RV afterload (pulmonary vasodilators, inodilators, RV “protec- CONCLUSION Heart, Lung and Vessels. 2014, Vol. 6 167 V. Zochios, et al. 168 tive” MV strategies) and maximizing RV performance (preload, inotropy, mechanical circulatory support) are the major components of ARHS management. Need for mechanical circulatory support, merits referral to specialized treatment centres, if there is insufficient local expertise or capacity. There is lack of definitive data regarding the management of ARHS in ICU patients, without pre-existing cardiopulmonary disease. Therefore, some recommendations may rely on lower level of evidence or expert opinion. Well-designed and adequately powered RCTs are required to estimate the prevalence of ARHS among critically patients receiving MV, improve the understanding of its mechanisms in the context of critical illness and evaluate the efficacy of therapy guided by invasive and non-invasive hemodynamic monitoring tools. REFERENCES 1. Greyson CR. Pathophysiology of right ventricular failure. Crit Care Med 2008; 36: S57-65. 2. Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 1997; 134: 479-487. 3. Bleeker GB, Steendijk P, Holman ER, Yu CM, Breithardt OA, Kaandorp TA, et al. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart 2006; 92: 19-26. 4. Alaverdian A, Cohen R. The Right Ventricle in critical illness. The Open Critical Care Medicine Journal 2010; 3: 38-42. 5. Kevin LG, Barnard M. Right ventricular failure. Contin Educ Anaesth Crit Care Pain 2007; 7: 89-94. 6. Zochios V, Keeshan A. Pulmonary embolism in the mechanically-ventilated critically ill patient: is it different? JICS 2013;14:36-44. 7. Reis Miranda D, Gommers D, Lachman B. Effect of mechanical ventilation on right ventricular afterload. In: Gullo A, eds. Anaesthesia, Intensive Care and Emergency Medicine. Trieste: Springer, 2006; 361-366. 8. Cecconi M, Johnston E, Rhodes A. What role does the right side of the heart play in circulation? Crit Care 2006; 10 (Suppl. 3): 6. 9. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease. I. Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008; 117: 1436-48. 10. Forrest P. Anaesthesia and right ventricular failure. Anaesth Intensive Care 2009; 37: 370-85. 11. Giovambattista R D. Hyperthyroidism as a reversible cause of right ventricular overload and congestive heart failure. Cardiovasc Ultrasound 2008; 6: 29. 12. Lahm T1, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY, Obeidat OS, et al. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol. 2010; 56: 1435-46. 13. Santambrogio L, Bianchi T, Fuardo M, Gazzoli F, Veronesi R, Braschi A, et al. Right ventricular failure after left ventricular assist device insertion: preoperative risk factors. Interact Cardiovasc Thorac Surg. 2006; 5: 37982. 14. Michael R, Pinsky M . Recent advances in the clinical application of heart- lung interactions. Curr Opin Crit Care 2002; 8: 26-31. 15. Jardin F, Gueret P, Dubourg O, Farcot JC, Margairaz A, Bourdarias JP. Two- dimensional echocardiographic evaluation of right ventricular size and contractility in acute respiratory failure. Crit Care Med 1985; 13: 952-6. 16. Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications and prognosis. Crit Care Med 2001; 29: 1551-5. 17. Snow RL, Davies P, Pontoppidan H, Zapol WM, Reid L. Pulmonary vascular remodeling in adult respiratory distress syndrome. Am Rev Respir Dis 1982; 126: 887-92. 18. Cook D, Attia J, Weaver B, McDonald E, Meade M, Crowther M. Venous thromboembolic disease: an observational study in medical-surgical intensive care unit patients. J Crit Care 2000; 15: 127-32. 19. Vieillard-Baron A, Page B, Augarde R, Prin S, Qanadli S, Beauchet A, et al. Acute cor pulmonale in massive pulmonary embolism: incidence, echocardiographic pattern, clinical implications and recovery rate. Intensive Care Med 2001; 27: 1481-6. 20. Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008; 4: 49-59. 21. Furian T, Aguiar C, Prado K, Ribeiro RV, Becker L, Martinelli N, et al. Ventricular dysfunction and dilation in severe sepsis and septic shock: relation to endothelial function and mortality. J Crit Care. 2012; 27: 319, e9-15. 22. Chan CM, Klinger JR. The right ventricle in sepsis. Clin Chest Med. 2008; 29: 661-76. 23. Markel TA, Wairiuko GM, Lahm T, Crisostomo PR, Wang M, Herring CM, et al. The right heart and its distinct mechanisms of development, function, and failure. J Surg Res 2007; 146: 304 -13. 24. Methvin AB1, Owens AT, Emmi AG, Allen M, Wiegers SE, Dries DL, et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest 2011; 139: 617-25. 25. Kevin LG, Barnard M. Right ventricular failure. Contin Educ Anaesth Crit Care Pain 2007; 7: 89-94. 26. Kucher N, Walpoth N, Wustmann K, Noveanu M, Gertsch M. QR in V1-an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolism. Eur Heart J. 2003; 24: 1113-9. 27. Miller GA, Sutton GC. Acute massive pulmonary embolism. Clinical and haemodynamic findings in 23 patients studied by cardiac catheterization and pulmonary arteriography. Br Heart J 1970;32:518-23. 28. Reems MM, Aumann M. Central venous pressure: principles, measurement, and interpretation. Compend Contin Educ Vet. 2012; 34: 1. 29. Greyson CR.Right heart failure in the intensive care unit. Curr Opin Crit Care 2012; 18: 424-31. 30. Matthay MA, Chatterjee K. Bedside catheterization of the Heart, Lung and Vessels. 2014, Vol. 6 Acute right heart syndrome in critical illness 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. pulmonary artery: risks compared with benefits. Ann Intern Med 1988; 109: 826. Goldstein JA, Barzilai B, Rosamond TL, Eisenberg PR, Jaffe AS. Determinants of hemodynamic compromise with severe right ventricular infarction. Circulation 1990; 82: 359-68. Zochios V, Wilkinson V. Assessment of intravascular fluid status and fluid responsiveness during mechanical ventilation in surgical and intensive care patients. JICS 2011; 12: 295-300. Wyler von Ballmoos M1, Takala J, Roeck M, Porta F, Tueller D, Ganter CC, et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care 2010; 14: 111. Daudel F, Tüller D, Krähenbühl S, Jakob SM, Takala J. Pulse pressure variation and volume responsiveness during acutely increased pulmonary artery pressure: an experimental study. Crit Care 2010; 14: R122. Michard F, Richards G, Biais M, Lopes M, Auler JO. Using pulse pressure variation or stroke volume variation to diagnose right ventricular failure? Crit Care 2010; 14: 451. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010; 23: 685-713; quiz 786-8. Jardin F, Dubourg O, Guéret P, Delorme G, Bourdarias JP. Quantitative two-dimensional echocardiography in massive pulmonary embolism: Emphasis on ventricular interdependence and leftward septal displacement. J Am Coll Cardiol 1987; 10: 1201-6. Hüttemann E. Transoesophageal echocardiography in critical care. Minerva Anestesiol. 2006; 72: 891-913. Kang DK, Thilo C, Schoepf UJ, Barraza JM Jr, Nance JW Jr, Bastarrika G, et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011; 4: 841-9. Mitoff PR, Beauchesne L, Dick AJ, Chow BJ, Beanlands RS, Haddad H, et al. Imaging the failing right ventricle. Curr Opin Cardiol. 2012; 27: 148-53. Lim W, Cook DJ, Griffith LE, Crowther MA, Devereaux PJ. Elevated cardiac troponin levels in critically ill patients: prevalence, incidence, and outcomes. Am J Crit Care 2006; 15: 280-88. Cepkova M, Kapur V, Ren X, Quinn T, Zhuo H, Foster E, et al. Clinical significance of elevated B-type natriuretic peptide in patients with acute lung injury with or without right ventricular dilatation: an observational cohort study. Ann Intensive Care 2011;1:18. Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010; 14: 169. Piazza G, Goldhaber SZ. The acutely decompensated right ventricle: pathways for diagnosis and management. Chest 2005; 128: 1836 -52. Marik PE, Baram M, Vahid B. Does the central venous pressure predict fluid resposiveness? A systematic review of the literature and the tale of seven mares. Chest 2008; 134: 172-8. Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013; 41: 1774-81. Mercat A, Diehl JL, Meyer G, Teboul JL, Sors H. Hemody- 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. namic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med. 1999; 27: 540-4. Giglioli C, Landi D, Cecchi E, Chiostri M, Gensini GF, Valente S, et al. Effects of ULTRAfiltration vs. DIureticS on clinical, biohumoral and haemodynamic variables in patients with deCOmpensated heart failure: the ULTRADISCO study. Eur J Heart Fail 2011; 13: 337-46. Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P, et al. Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Crit Care Med 2004; 32: 1035-40. Luckner G, Mayr VD, Jochberger S, Wenzel V, Ulmer H, Hasibeder WR, et al. Comparison of two dose regimens of arginine vasopressin in advanced vasodilatory shock. Crit Care Med 2007; 35: 2280-5. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008; 117: 1717-31. Le Tulzo Y, Seguin P, Gacouin A, Camus C, Suprin E, Jouannic I, et al. Effects of epinephrine on right ventricular function in patients with severe septic shock and right ventricular failure: a preliminary descriptive study. Intensive Care Med. 1997; 23: 664-70. Kerbaul F, Rondelet B, Demester JP, Fesler P, Huez S, Naeije R, et al. Effects of levosimendan versus dobutamine on pressure load-induced right ventricular failure. Crit Care Med 2006; 34: 2814-9. Morelli A, Teboul JL, Maggiore SM, Vieillard-Baron A, Rocco M, Conti G, et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med 2006; 34: 2287-93. Pathak A, Lebrin M, Vaccaro A, Senard JM, Despas F. Pharmacology of levosimendan: inotropic, vasodilatory and cardioprotective effects. J Clin Pharm Ther. 2013; 38: 341-9. Summerfield DT, Desai H, Levitov A, Grooms DA, Marik PE. Inhaled nitric oxide as salvage therapy in massive pulmonary embolism: a case series. Respir Care. 2012; 57: 444-8. Clapp LH, Finney P, Turcato S, Tran S, Rubin LJ, Tinker A. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol 2002; 26: 194-201. Radermacher P, Santak B, Wust HJ, Tarnow J, Falke KJ. Prostacyclin and right ventricular function in patients with pulmonary hypertension associated with ARDS. Intensive Care Med 1990; 16: 227-32. Karakitsos D, Papanikolaou J, Karabinis A, Alalawi R, Wachtel M, Jumper C, Alexopoulos D, et al. Acute effect of sildenafil on central hemodynamics in mechanically ventilated patients with WHO group III pulmonary hypertension and right ventricular failure necessitating administration of dobutamine. Int J Cardiol. 2013; 167: 848-54. Schmitt JM, Vieillard-Baron A, Augarde R, Prin S, Page B, Jardin F. Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: impact on right ventricular outflow impedance evaluated by pulmonary artery Doppler flow velocity measurements. Crit Care Med. 2001; 29: 1154-8. Hakim TS, Michel RP, Chang HK. Effect of lung inflation on pulmonary vascular resistance by venous and arterial occlusion. J Appl Physiol 1982; 53: 1110-5. Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child 1999; 80: 475-80. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Heart, Lung and Vessels. 2014, Vol. 6 169 V. Zochios, et al. 170 64. 65. 66. 67. 68. 69. 70. 71. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342: 1301-8. Balanos GM, Talbot NP, Dorrington KL, Robbins PA. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol 2003; 94: 1543-51. Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009; 35: 1850-8. Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care 2011; 15: 243. Stratta C, Lavezzo B, Ballaris MA, Panio A, Crucitti M, Andruetto P, et al. Extracorporeal membrane oxygenation rescue therapy in a case of portopulmonary hypertension during liver transplantation: a case report. Transplant Proc. 2013; 45: 2774-5. Tiruvoipati R, Botha JA, Pilcher D, Bailey M. Carbon dioxide clearance in critical care. Anaesth Intensive Care 2013; 41: 157-62. Teboul JL, Monnet X, Richard C. Weaning failure of cardiac origin: recent advances. Crit Care 2010; 14: 211. Boeken U, Feindt P, Litmathe J, Kurt M, Gams E. Intraaortic balloon pumping in patients with right ventricular insufficiency after cardiac surgery: parameters to predict failure of IABP Support. Thorac Cardiovasc Surg. 2009; 57: 324-8. Arafa OE, Geiran OR, Andersen K, Fosse E, Simonsen S, 72. 73. 74. 75. 76. 77. Svennevig JL. Intraaortic balloon pumping for predominantly right ventricular failure after heart transplantation. Ann Thorac Surg. 2000;70:1587-93. Nordhaug D, Steensrud T, Muller S, Husnes KV, Myrmel T. Intraaortic balloon pumping improves hemodynamics and right ventricular efficiency in acute ischemic right ventricular failure. Ann Thorac Surg. 2004; 78: 1426-32. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287-96. Belohlavek J, Rohn V, Jansa P, Tosovsky J, Kunstyr J, Semrad M, et al.Veno-arterial ECMO in severe acute right ventricular failure with pulmonary obstructive hemodynamic pattern. J Invasive Cardiol. 2010; 22: 365-9. Gregoric ID, Chandra D, Myers TJ, Scheinin SA, Loyalka P, Kar B. Extracorporeal membrane oxygenation as a bridge to emergency heart-lung transplantation in a patient with idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2008; 27: 466-8. Moazami N, Pasque MK, Moon MR, Herren RL, Bailey MS, Lawton JS, et al. Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant. 2004; 23: 1371-5. Berman M, Tsui S, Vuylsteke A, Klein A, Jenkins DP. Lifethreatening right ventricular failure in pulmonary hypertension: RVAD or ECMO? J Heart Lung Transplant 2008; 27: 1188-9. Cite this article as: Zochios V, Jones N. Acute right heart syndrome in the critically ill patient. Heart, Lung and Vessels. 2014; 6(3): 157-170. Source of Support: Nil. Disclosures: None declared. Heart, Lung and Vessels. 2014, Vol. 6 ORIGINAL ARTICLE Heart, Lung and Vessels. 2014; 6(3): 171-179 Atrial fibrillation after isolated coronary surgery. Incidence, long term effects and relation with operative technique C. Rostagno1, C. Blanzola2, F. Pinelli3, A. Rossi3, E. Carone2, P.L. Stefàno2 1 Department of sperimental and clinical medicine, University of Florence; 2Heart Surgery Unit AOU Careggi, Florence; Cardio Anesthesiologic Unit, AOU Careggi, Florence 3 Heart, Lung and Vessels. 2014; 6(3): 171-179 ABSTRACT Introduction: Postoperative atrial fibrillation after isolated coronary revascularization has been associated with increased morbidity and mortality. Aim of present investigation was to evaluate incidence of postoperative atrial fibrillation and its prognostic role in patients undergoing isolated coronary artery by-pass and disclose possible differences between off-pump and cardiopulmonary assisted revascularization. Methods: Prospective cohort study of 229 patients undergoing isolated coronary artery by-pass at a tertiary heart surgery Centre. Off-pump treated patients were significantly older (70.5 vs 64.9 years, p<0.001). No other baseline differences were found. Patients who developed postoperative atrial fibrillation were followed up for an average period of 2 years. Results: Post-operative occurred in 56/229 (24.1% after cardiopulmonary and 24.6% after off–pump coronary artery by-pass). Left atrium diameter was the only independent predictive factor (odds ratio =1.15, 95% confidence interval 1.02-1.30, p<0.001). All patients with postoperative atrial fibrillation were treated and discharged in sinus rhythm, in 6/56 recurred, only in one persisted. One patient died during follow up. No stroke was recorded. Conclusions: After isolated surgical revascularization, atrial fibrillation occurred in 24% without differences related to operative technique. Recurrence of atrial fibrillation occurred in 6/56 patients (10.7%) however only in 1 persisted. Early and late mortality did not show relation with post-operative atrial fibrillation probably due to immediate treatment with recovery of sinus rhythm before discharge. Keywords: atrial fibrillation, cardiac revascularization, stroke, mortality. INTRODUCTION Atrial fibrillation occurs in 20-30% after surgical cardiac revascularization (1). Several studies suggest that postoperative Corresponding author: Department of sperimental and clinical medicine University of Florence Viale Morgagni, 85 50134 Florence e-mail: carlo.rostagno@unifi.it atrial fibrillation (POAF) is associated with an increased duration of hospitalization, early and long term morbidity and mortality (2, 3). Off-pump coronary artery by-pass (CABG) grafting (OP-CABG) has been hypothesized to decrease incidence of POAF (4, 5); however contrasting results has been reported (6, 7). Aim of present prospective investigation was to evaluate the incidence of POAF in patients undergoing isolated surgical revascularization in a Heart, Lung and Vessels. 2014, Vol. 6 171 C. Rostagno, et al. 172 tertiary heart surgery centre. Patients with POAF were followed for an average period of 2 years to assess the recurrence rate of the arrhythmia and its prognostic role on early and late risk of stroke and mortality. Finally, the role of cardiopulmonary by-pass (CPB) surgical revascularization (CPB-CABG) and OP-CABG on POAF was evaluated. METHODS Study population. Among 822 patients who underwent heart surgery between Jan 1 2009 and Dec 31 2009 in a tertiary heart surgery Centre, 229 patients in sinus rhythm on hospital admission (179 males, 50 females) underwent isolated CABG (138 - OP-CABG, 91 - CPB-CABG). Patients with atrial fibrillation, hyperthyroidism or scheduled for Maze procedure were excluded from the study. In patients undergoing isolated CABG, bipolar Maze procedure was usually planned for subjects with persistent or frequent episodes of paroxysmal atrial fibrillation. Finally, patients with more than mild valvular disease and creatinine clearace <30 ml/min were excluded. Echocardiographic evaluation was performed within 48 hours before surgery using a Sequoia Acuson Instrument (Siemens Medical Solution, Mount View, CA, USA). Echocardiography was performed according to the guidelines of the American Society of Echocardiography (8). Clini- Table 1 - Clinical and echocardiographic characteristics of patients included in the study mean (standard deviation). Overall (229) OP-CABG (138) CPB- CABG (91) p Age (SD) 68.4 (9.2) 70.5 (8.6) 64.9 (9.0) 0.005 Sex M/F 179/50 101/37 78/13 0.06 Left atrium diameter (mm) 39.7 (4.6) 40.2 (4.6) 39.3 (4.5) 0.25 Left vetricular ejection fraction % 51.1(9.9) 50.7 (9.7) 51.6 (10.3) 0.66 Hypertension (%) 166 (72.5) 105 (76.1) 61 (67.0) 0.17 Hystory of atrial fibrillation (%) 6 (2.6) 5 (3.6) 1 (1.1%) 0.40 ACE Inhibitors or AT1 Blockers 169 107 62 0.12 Statins 160 99 61 0.46 ` Blockers 118 74 44 0.14 P.O. Heart Rate (SD) 84.9 (19.9) 80.5 (12.9) 85.3 (14.3) 0.06 Systolic Blood Pressure mmHg (SD) 137 (23.5) 137.5 (19.5) 139 (20.0) 0.65 Diastolic Blood Pressure mmHg (SD) 68.5 (12.1) 66.9 (13) 70.8 (9.8) 0.07 Transient pace -maker stimulation 8 5 3 1 P.O bleeding (%)* 15 8 7 0.59 Hemodynamic impairment (%)** 16 11 5 0.61 Other (%) 11 6 5 0.75 Atrial Fibrillation (%) 56 (24,4) 34 (24.6) 22 (24.1) 0.9 Length of Hospitalization (Days) 5.7 (4.5) 5.7 (4.8) 5.6 (4.1) 0.94 *Bleeding requiring transfusion at least 2 units of packed red blood cells or surgical revision. **Hemodynamic deterioration with the need to infuse amines. OP = off pump; CPB = cardipulonary-by pass; CABG = coronary artery by-pass; SD= standard deviation. Heart, Lung and Vessels. 2014, Vol. 6 Atrial fibrillation after CABG 173 Table 2 - Clinical diagnosis, number of diseased vessels and grafts performed. Clinical diagnosis Overall 229 OP-CABG 138 CPB-CABG 91 p Chronic CAD 167 98 69 0.67 ACS 59 39 20 0.35 3 1 2 0.56 Elective surgery STEMI 160 96 64 0.64 Urgency 61 37 24 0.9 Emergency 8 5 3 0.86 3 vessels 99 64 35 0.22 3 vessel + LMC 58 32 26 0.65 LMC 35 20 15 0.71 2 vessels 17 10 7 0.9 2 vessels + LMC 8 5 3 0.85 1 vessel 10 7 5 0.79 Diseased vessels By-pass conduit LIMA 18 11 7 0.87 LIMA+ RIMA 50 29 21 0.74 LIMA + saph 94 61 33 0.27 LIMA+ RIMA + saph 67 37 30 0.37 OP = off pump; CABG = coronary artery by-pass; CAD = coronary artery disease; ACS = acute coronary syndrome; STEMI = elevated ST acute myocardial infarction ; LMC = left main coronary; LIMA = left internal mammary artery; RIMA = right internal mammary artery; saph = saphene vein. cal and echocardiographic characteristics of patients are reported in Table 1. In Table 2 clinical diagnosis, indications for surgery (elective, urgency/emergency), the number of diseased vessels and graft performed in the groups under investigation are reported. Thirty clinical and echocardiographic variables were considered to evaluate a relationship with occurrence of POAF. After surgery all patients were continuously monitored electrocardiography (ECG), blood pressure, non-invasive oxygen saturation for at least the first 48 hours. ECG monitoring, both at bed and by telemetry, was maintained until discharge. Transient electric stimulation through epicardic wires was used for severe bradycardia or atrio-ventricular (AV) block until restoration of heart rhythm. All symptomatic ar- rhythmic episodes or asymptomatic atrial fibrillation lasting more than 15 minutes at ECG monitoring were considered as POAF and included in the analysis. Patients who did not recover sinus rhythm (SR) within 30 minutes were usually treated with intravenous amiodarone (300 mg in 1 hour followed by 900 mg/24 h e.v. continuous infusion) to control heart rate. Electrical cardioversion was considered when sinus rhythm was not restored within 24 hours after the beginning of pharmacological treatment. Amiodarone was continued for 3 months after discharge. Perioperative complications including bleeding needing transfusion of at least 2 units of packed red blood cells and/or surgical revision, severe hypotension requiring amines (norepinephrine, epinephrine, dobutamine or do- Heart, Lung and Vessels. 2014, Vol. 6 C. Rostagno, et al. 174 pamine), and new onset AV block or severe bradycardia requiring electrical stimulation were recorded. Postoperative pericardial inflammation was diagnosed in the presence of pericardial rubs and/or ECG or echocardiogram signs of pericardial involvement. In the end, duration of hospitalization was examined. All patients was discharged to rehabilitation facilities. The study was approved by the ethic committee of our Institution and all participants gave their informed consent. Follow-Up. Only patients with POAF were followed-up and entered the study. Followup visit were scheduled after 3, 12 and 24 months. Holter monitoring was performed every 3 months during the first year and thereafter every 6 months. Follow-up was closed on December 31 2012. No patient was lost at follow-up. Primary end point of the study was the evaluation of recurrence of atrial fibrillation and related hospitalization; secondary end points were all cause hospitalization and mortality. Finally we evaluated the role of surgical technique (CPB-CABG vs OP-CABG) on POAF. Statistical Analysis. Data were described as mean and standard deviation (SD) for continuous variables and as number and percent for categorical variables. Preoperative and operative patient characteristics were compared according to the occurrence of postoperative AF by means of the Student t test or Fisher exact test for continuous and categorical variables, respectively, or finally by ANOVA. Multivariate logistic regression analysis was used to evaluate independent risk factors for atrial fibrillation. RESULTS In-Hospital Outcomes. Overall incidence of POAF during hospitalization was 24.4% (56 of 229 patients), 38 males and 18 females. 1/229 patients died after surgery. Patients who developed AF after surgery were older than ones in stable sinus rhythm (70.5 vs 64.9 years, p=0.005). POAF was not related to clinical indication to surgery (elective vs urgency/emergency), number of diseased vessels or graft performed (Table 3). Postoperative troponin release did not differ between two groups. In patients with POAF left ventricular ejection fraction was not significantly lower than in sinus rhythm group (49% vs 51%). Two out of six patients with paroxysmal atrial fibrillation before surgery developed POAF. The use of beta-blockers, angiotensin converting enzyme (ACE) inhibitors/ angiotensin A1 receptor (AT1) blockers and statins did not influence the prevalence of postoperative AF. Patients with AF did not show a different prevalence of intra-aortic balloon pump use or treatment with vasopressors or inotropic drugs after surgery. Transient electric stimulation after surgery was needed in 2 patients with POAF and in 3 who did not develop arrhythmias. No permanent pacing was required. Multivariate analysis revealed that only antero-posterior left atrium diameter was associated with an increased risk of POAF (odds ratio = 1.15; 95% confidence interval (CI) [1.02, 1.30], p<0.001) (Table 4). The frequency of POAF was not statistically different between patients undergoing OP-CABG and those undergoing cardiopulmonary by-pass (24.6% for OPCABG vs 24.1% for CPB-CABG.) Table 3 reports the relative number of elective in comparison to urgency/emergency procedures, and the number of grafts conduits employed in the two groups. Patients with POAF undergoing OP-CABG were on average 7 years older than CBP- CABG (74.3 vs 67 years, p<0.001). There were no other significant differences between the two groups. All patients with atrial fibrillation were successfully treated and discharged in sinus rhythm. Length of hospitalization Heart, Lung and Vessels. 2014, Vol. 6 Atrial fibrillation after CABG was on average 2 days longer in patients with POAF after CPB-CABG. Follow-up results. The 56 patients with postoperative atrial fibrillation were followed-up for a median of 685 days. 6 male patients, had recurrence of atrial fibrillation (10.7%). Age was not significant dif- ferent in those patients (average age 72.5 vs 71.3 years). Among those patients, three underwent OP-CABG, and the others CPBCABG. Preoperative left ventricular ejection fraction was not different in patients with AF in comparison to patients without recurrence (48% vs 49%), while mean left Table 3 - Comparison of clinical, echocardiographic characteristics and conduits used for grafting between patients with and without POAF according to surgical technique. Mean (standard deviation) POAF (56) OP (34) CPB (22) p 74.3 (6.7) 67 (9.5) 0.001 22/12 16/6 0.57 Left atrium diameter (mm) 43.4 (4.5) 40.3 (5.3) 0.001 Left ventricular ejection fraction % 48 (10.2) 50.2 (9.1) 0.46 28 12 0.07 Age. Years Sex M/F Hypertension Hystory of atrial fibrillation 2 0 0.51 ACE Inhibitors or AT1 Blockers 27 10 0.08 Statins 22 13 0.77 ` Blockers 22 13 0.77 P.O. Heart Rate (SD) 82.8 (16.9) 85.35 (16.01) 0.51 Sistolic Blood Pressure mmHg (SD) 139.3 (16.1) 134 (17.8) 0.27 66. 6 (9.4) 69.8 (11.1) 0.23 2 0 0.51 Diastolic Blood Pressure mmHg (SD) Transient pace-maker stimulation P.O bleeding (%)* 3 5 0.14 Hemodynamic impairment (%)** 3 0 0.46 15 0.77 CLINICAL CONDITION COPD 1 Chronic CAD 21 Acute Coronary Syndrome 11 7 0.8 STEMI 1 1 1 Left Internal Mammary Artery 2 3 0.37 LIMA + RIMA 10 7 0.9 LIMA + SAPH. 18 9 0.78 LIMA+RIMA +Saphen vein 4 3 0.9 5.9 (3.6) 7. 5 (6.8) 0.25 By-Pass Conduit Length of Hospitalization (Days) *Bleeding requiring transfusion at least 2 units of packed red blood cells or surgical revision. ** Hemodynamic deterioration with the need to infuse amines. POAF = postoperative atrial fibrillation; OP = off pump; CPB = cardipulonary-by pas; COPD = chronic obstructive pulmonary disease; CAD = coronary artery disease; ACS = acute coronary syndrome; STEMI = elevated ST acute myocardial infarction ; LMC= left main coronary; LIMA = left internal mammary artery, RIMA = right internal mammary artery, SAPH = saphen vein; SD = stabdard deviation. Heart, Lung and Vessels. 2014, Vol. 6 175 C. Rostagno, et al. 176 Table 4 - Risk factors for POAF. Logistic regression analysis. age sex election vs urgencyemergency LA diameter `-blockers ACE inhibitors statins heart rate LV ejection fraction Systolic BP Diastolic BP troponin peak serum potassium OD 95% CI 0,95 1.08 1.18 1,15 2,47 0,52 0,41 1,00 0.99 1,02 0,96 0,96 1,20 0,90 1,01 0.95 -1.18 0,44 - 2,21 1,02 - 1,30 0,85 - 7,14 0,15 - 1,76 0-13 – 1.2 0.98 – 1.03 0.94 – 1.05 0.99 – 1.04 0.92 - 1.01 0.85 – 1.08 0.89 – 1.64 p 0.14 0.18 0.72 0.01 0.09 0.29 0.12 0.49 0.97 0.11 0.15 0.51 0.22 OD = odds ratio; CI = confidence interval; LA = left atrium; ACE = angiotensin-converting-enzyme; LV = left ventricle; BP = blood pressure. atrium anterior-posterior diameter was respectively 42 mm and 40 mm. On average, the AF recurrences occurred within 60 days after discharge. Amiodarone treatment was successful in 3 patients, electric cardioversion in one case. In one patient sinus rhythm recovered spontaneously. Ultimately, in the last patient sinus rhythm could not be restored. At the end of follow up only one patient died not for cardiovascular cause (lung cancer). DISCUSSION Incidence of atrial arrhythmias after cardiac surgery has been reported to range from 10 to 65% (9, 10). According to a large multi-centre study, POAF after CABG occurs in near 30% (11). Several factors, including type of surgical procedure, patient demographics, criteria used for diagnosis and methods of ECG monitoring, may account for the wide range of POAF incidence reported in literature. Several mechanisms are involved in the pathogenesis of POAF. Dispersion in atrial refractoriness induces multiple local re-entry wavelets; therefore, atrial fibrillation may be induced by several factors. Among these: trauma from surgical dissection and manipulation, myocardial ischemic damage, an exaggerated local inflammatory response with or without pericarditis, an elevation in atrial pressure from post-operative ventricular stunning a chemical stimulation due to postoperative support with catecholamine and other inotropic agents, a reflex sympathetic activation from volume loss, anemia or pain, parasympathetic activation, fever from atelectasis or infection, hypoglycaemia, metabolic and electrolyte imbalance, fluid overload, prolonged post operative electrical stimulation. Cardiopulmonary by-pass related hemodynamic changes may induce intraoperative atrial ischemia that has been hypothesized to play a role in the development of POAF. Evidence supporting an association between AF after CABG surgery and late mortality is conflicting. Few data of patients with POAF after hospital discharge are available. Almassi et al. (3) reported at 6 months after surgery a significantly higher mortality in AF patients compared Heart, Lung and Vessels. 2014, Vol. 6 Atrial fibrillation after CABG with patients without AF (9.4% vs 4.2%). Villareal et al. (2) showed in 6475 patients undergoing first isolated CABG that POAF was associated with at increased risk of death (odds ratio =1.5; 95% CI [1.3, 1.8]). In this study, cumulative survival rate at 1 and 4 years was 87% and 74% in POAF patients versus 94% and 87% for non-AF population. In more than 8500 isolated CABG patients a significantly increased risk of death was observed among those who developed postoperative AF compared with those who did not (odds ratio =1.2; 95% CI, 1.1 to 1.3) (11). Patients affected by postoperative AF had an increased 1-year mortality (4.6% versus 2.0%), and AF was confirmed to independently predict late mortality (hazard ratio, 1.7; 95% CI [1.2, 2.5]) (10). Results from present investigation do not support an association of POAF with an increase of late mortality in patients undergoing surgical revascularization. Only one patient died at 2 years follow-up and not for cardiovascular cause. Restoration of sinus rhythm in all patients during hospitalization and low recurrence rate may have significantly decreased the risk of mortality, in particular due to stroke or complications of oral anticoagulant treatment. Decreased risk of ischemic damage in beating heart CABG has been suggested to reduce incidence of POAF. Initial favourable results (6, 7) has not been confirmed by other authors (4, 12). Siebert et al (5) during the postoperative intensive care unit stay reported a 9.8% rate of POAF in patients after CPB-CABG, 10.2% after OP-CABG, and 21% after CABG combined with valve replacement. A recent meta-analysis suggested a decreased incidence of AF in OPCABG although overall mortality was not affected (13). A not significant difference in the incidence of POAF between the two techniques was reported by several other stud- ies (14, 15). Two randomized, controlled trials and one large scale concurrent cohort study addressed the issue of beatingheart CABG. Ascione et al (7) found a significantly lower rate of postoperative AF in the OP-CABG group (11.0%) than in the CBP-pump CABG group (45.0%) in 200 patients who had been randomized to undergo CABG either with or without CPB. A significant difference in postoperative AF favouring the OP-CABG group (21.2%) compared to the on-pump CABG group (6.3%) was reported by Hernandez et al (16). In contrast, in 281 patients randomized to CABG with or without CPB, Van Dijk et al (9) reported no difference in the rate of postoperative AF. In patients undergoing emergency revascularization for acute coronary syndromes off-pump surgery vs CPB surgery was performed in patients with more severe clinical conditions: OP-CABG patients were more frequently in cardiogenic shock, had an impaired renal function, a log EUROSCORE >20 or a left ventricular ejection fraction <30% (17). Overall survival and event rate, however, were similar at 5 five year follow-up. Postoperative AF occurred in 30.2% patients undergoing CPB-CABG vs 29.3% in OP-CABG surgery while the incidence of stroke was two-fold in the former group (6.7 vs 2.5%, p<0.035). Noteworthy the incidence of AF was two folds in patients with cardiogenic shock undergoing CPB versus OP surgery (62.5 vs 39.8%), with a significant increase in the number of stroke (33.3 vs 9.6%, p<0.009). Recently a TnI serum concentration >0.901 ng/ml at ICU admission has been identified as cutoff value for prediction of AF in patients undergoing elective CABG (18). Patients with serum TnI > of 0.901 ng/ml showed an 11.5 times increased risk for the onset of AF after elective CABG. In present investigation no significant relation was found between TnI serum concentration after sur- Heart, Lung and Vessels. 2014, Vol. 6 177 C. Rostagno, et al. 178 gery and the risk of AF (odds ratio =0,9695% CI [0,85, 1,08], p=0.17). In our experience, the incidence of POAF resulted not significantly different in patients undergoing OP-CABG in comparison to CPB-CABG. The two groups were comparable for severity of coronary disease, number of grafts performed and clinical presentation (urgent versus elective surgery). However, mean age of patients undergoing OP-CABG was on average 7 years older in comparison to patients treated with CPB-CABG. erative AF is associated with an increased late mortality, rate of stroke or rehospitalization. Restoration of sinus rhythm before hospital discharge may have significantly limited the negative prognostic effects of post operative atrial fibrillation. At present, the guidelines of the American College of Chest Physician state that OPCABG cannot be recommended to decrease postoperative AF because of conflicting results resulted from randomized controlled trials or large-scale concurrent cohort studies (20). Limitations The present study was limited by its observational nature, by a relative short followup period (2 years), and by the low number of patients investigated. In addition, patients were not randomized to either treatment. Otherwise the short time of enrolment (1 year), the similar characteristics of the two groups, with the cited exception of age, and the limited number of operators decreased the risk of non homogeneity of the population under investigation. The low number of recurrences occurred, similarly to other studies, may be related to potential bias due to the reliance on self-reporting for follow-up cardiac rhythm data. Although scheduled Holter monitoring in our investigation did not reveal paroxysmal episodes of atrial fibrillation, only continuous monitoring systems may provide definitive data. Similarly, the use of questionnaires and clinical examination during follow-up may not accurately identify paroxysmal episodes of AF and may potentially have underestimated the incidence of the arrhythmia recurrences. REFERENCES CONCLUSION Despite the reported limitations, our study does not support the hypothesis that postop- 1. Hravnak M, Hoffman LA, Saul MI, Zullo TG, Whitman GR. Resource utilization related to atrial fibrillation after coronary artery bypass grafting. Am J Crit Care. 2002; 11: 228-38. 2. Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004; 43: 742-48. 3. Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997; 226: 501-11. 4. Buffolo E, de Andrade JCS , Branco JN, Teles CA, Aguiar LF, Gomes WJ. Coronary artery bypass grafting without cardiopulmonary bypass. Ann Thorac SurAg 1996; 61: 63-6. 5. Siebert J, Anisimowicz L, Lango R, Rogowski J, Pawlaczyk R, Brzezinski M, et al. Atrial fibrillation after coronary artery bypass grafting: does the type of procedure influence the early postoperative incidence? Eur J Cardiothorac Surg. 2001; 19: 455-9. 6. Stamou SC, Dangas G, Hill PC, Pfister AJ, Dullum MK, Boyce SW, et al. Atrial fibrillation after beating heart surgery. Am J Cardiol. 2000; 86: 64-7. 7. Ascione R, Caputo M, Calori G, Lloyd CT, Underwood MJ, Angelici GD. Predictors of atrial fibrillation after conventional and beating heart coronary surgery: a prospective, randomized study. Circulation. 2000; 102: 1530-5. 8. Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ ASE/ACEP/ASNC/SCAI/SCC/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance. Endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Soc Echocardiogr. 2007; 20: 787-805. 9. van Dijk D, Nierich AP, Jansen EW, Nathoe HM, Suyker WJ, Diephuis JC, at al. Octopus Study Group. Early outcome after off-pump versus on-pump coronary bypass surgery: results from a randomized study. Circulation 2001; 104: 1761-6. Heart, Lung and Vessels. 2014, Vol. 6 Atrial fibrillation after CABG 10. Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization: Multicenter Study of Perioperative Ischemia Research Group. JAMA. 1996; 276: 300-6. 11. Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004; 291: 1720-9. 12. Cohn WE, Sirois CA, Johnson RG. Atrial fibrillation after minimally invasive coronary artery bypass grafting: a retrospective, matched study. J Thorac Cardiovasc Surg 1999; 117: 298-301. 13. Moller CH, Penninga L, Wetterslev J, Steinbruchel DA. Offpump versus on pump coronary artery bypass grafting for ischaemic heart disease. Cochrane Database Syst Rev. 2012 Mar 14; 3: CD007224. 14. Abreu JE, Reilly J, Salzano RP, Khachane VB, Jekel JF, Clyne CA. Comparison of frequencies of atrial fibrillation after coronary artery bypass Am J Cardiol. 1999; 83: 775-6. 15. Banach M, Goch A, Misztal M, Rysz J, Zaslonka J, Goch JH, et al. Relation between postoperative mortality and atrial fibrillation before surgical revascularization: 3-year followup. Thorac Cardiovasc Surg. 2008; 56: 20-3. 16. Hernandez F, Cohn WE, Baribeau YR, Tryzelaar JF, 17. 18. 19. 20. Charlesworth DC, Clough RA, et al. In-hospital outcomes of off-pump versus on-pump coronary artery bypassprocedures: a multicenter experience. Ann Thorac Surg. 2004; 78: 1528-34. Rastan AJ, Eckenstein JI, Hentschel B, Funkat AK, Gummert JF, Doll N, et al. Emergency coronary artery bypass graft surgery for acute coronary syndrome: beating heart versus conventional cardioplegic cardiac arrest strategies. Circulation. 2006; 114 (Suppl. I): 477-85. Leal JC, Petruccic O, Godoya MF, Brailea ML. Perioperative serum troponin I levels are associated with higher risk for atrial fibrillation in patients undergoing coronary artery bypass graft surgery. Intern CardioVascular and Thorac. Surg. 2012; 14: 22-25 Mahoney EM, Thompson TD, Veledar E, Williams J, Weintraub WS. Cost-effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J Am Coll Cardiol. 2002; 40: 737-45. Creswell LL, Alexander JC, Ferguson B, Lisbon A, Fleisher LA. Intra operative interventions: American College of Chest Physicians Guidelines for the Prevention and Management of Postoperative Atrial Fibrillation After Cardiac Surgery. Chest. 2005; 128 (Suppl. 2): 28-35. Cite this article as: Rostagno C, Blanzola C, Pinelli F, Rossi A, Carone E, Stefàno PL. Atrial fibrillation after isolated coronary surgery. Incidence, long term effects and relation with operative technique. Heart, Lung and Vessels. 2014; 6(3): 171-179. Source of Support: Nil. Disclosures: None declared. Heart, Lung and Vessels. 2014, Vol. 6 179 ORIGINAL ARTICLE Heart, Lung and Vessels. 2014; 6(3): 180-186 180 Acute myocardial infarction associated to DPP-4 inhibitors J.P.L. Nunes1,2, J.D. Rodrigues2, F. Melão2 1 Faculdade de Medicina da Universidade do Porto, Portugal; 2Department of Cardiology, Hospital Sao Joao, Porto, Portugal Heart, Lung and Vessels. 2014; 6(3): 180-186 ABSTRACT Introduction: Diabetes mellitus is associated with cardiovascular disease. Anti-diabetic therapy has a limited capability (if any) of changing the incidence of either death or major cardiovascular disease, and cardiovascular safety concerns have been raised. We aimed at identifying episodes of acute myocardial infarction associated to a relatively new class of drugs, dipeptidyl peptidase-4 inhibitors. Methods: Retrospective study: from 954 admissions (15 month period) in the coronary care unit, we selected 200 admissions corresponding to 196 patients with myocardial infarction and diabetes. 35 of these patients were receiving therapy with dipeptidyl peptidase-4 inhibitors (the vast majority, in association to metformin). We evaluated the peak plasma cardiac troponin I as the main study parameter. Results: Patients on dipeptidyl peptidase-4 inhibitors therapy had a mean peak cardiac troponin plasma level of 50.2±121.3 ng/ml (n=35), the corresponding value for insulin being 39.2±108.4 ng/ml (n=56), for metformin the value was 45.8±97.3 ng/ml (n=93) and for sulfonylureas, 42.4±77.7 ng/ml (n=52). None of these values differed significantly from the corresponding control group of patients not taking each class of drug. The linear regression study also yielded a negative result relating therapy with dipeptidyl peptidase-4 inhibitors and peak troponin values. Acute myocardial infarctions associated to dipeptidyl peptidase-4 inhibitors varied widely in the clinical characteristics of the patients. Conclusions: We found no evidence that peak plasma troponin I was different between patient with acute myocardial infarction and use of dipeptidyl peptidase-4 inhibitors when compared to cases not under such therapy. Keywords: acute myocardial infarction, diabetes mellitus, dipeptidyl peptidase-4 inhibitors, metformin, troponin. INTRODUCTION Diabetes mellitus is a highly prevalent disease that acts as a cardiovascular risk factor (1). The presence of diabetes mellitus is associated with an increase in the mortality rate of patients, both with or without a previous myocardial infarction (2). Diabetes mellitus has been shown to be associated to an increased incidence of coronary Corresponding author: José Pedro L. Nunes Faculdade de Medicina da Universidade do Porto Alameda Prof. Hernani Monteiro 4200 Porto, Portugal e-mail: jplnunes@med.up.pt artery disease and stroke (3). Clinical trials have shown anti-diabetic therapies to have very limited, if any, capability to change the incidence of either death or major cardiovascular disease, such as myocardial infarction or stroke (4). Issues of cardiovascular safety associated to anti-diabetic therapy have been put forward (5). Dipeptidyl peptidase-4 inhibitors (DPP4 inhibitors) are a relatively new class of anti-diabetic drugs that have been shown to decrease glycated hemoglobin, either if used alone or in association to other drugs such as metformin. In patients with myocardial infarction, the Heart, Lung and Vessels. 2014, Vol. 6 Myocardial infarction and DPP-4 inhibitors presence of diabetes mellitus is relatively common. In the present investigation, we aimed to characterize episodes of acute myocardial infarction associated to the use of DPP-4 inhibitors, as well as to other antidiabetic drugs. For that purpose, data from the admissions that took place during 15 months in an acute coronary care unit were retrospectively evaluated. Peak plasma cardiac troponin I was the major parameter under study, since plasma troponin provides an estimate of the importance of the myocardial injury in myocardial infarction (6). METHODS The present study was retrospective. From all patients admitted to an intensive coronary care unit from January 2011 to March 2012, patients with both acute myocardial infarction and diabetes mellitus were identified. A patient was considered to have diabetes mellitus if anti-diabetic therapy was being taken, if the diagnosis had been previously established on the basis of current recommendations (7) or if glycated hemoglobin greater than 6.5% (7) was present at admission. Acute myocardial infarction was diagnosed following the recommendations in use (8). Patients with in-hospital acute myocardial infarction were excluded. Patients who were initially admitted to another hospital, and who were later transferred into our institution were only included if the peak value for plasma troponin I could be clearly identified. Data on previous anti-diabetic drug use was searched in the electronic file(s) corresponding to each patient. Peak plasma cardiac troponin I levels was also searched in the corresponding electronic files. Additional data were obtained for each patient on the following parameters: presence of ST segment elevation in the electrocardiogram; previous history of myocardial infarction; previous coronary revascularization, either percutaneous or surgical; primary coronary angioplasty in the current episode; plasma creatinine at admission. Peak cardiac troponin levels in patients under anti-diabetic therapy with DPP-4 inhibitors were compared to the corresponding values for patients under no such therapy. The same comparison was carried out regarding insulin, metformin and sulfonylureas. Troponin I was measured using the ARCHITECT STAT system, of Abbott Diagnostics (Abbott Park, Illinois, USA). The 99th percentile of troponin I in a normal population with this assay was established at 0.012 ng/ml. The present protocol was approved by the ethics committee of our institution. Statistical Methods. Data are presented as arithmetic means and standard deviations. Pairs of means were compared using Mann Whitney U test. Linear regression analysis was carried out, taking peak plasma troponin I as dependent variable, and age, sex, plasma creatinine at admission, presence of ST segment elevation and use of DPP-4 inhibitors, use of metformin, use of insulin and use of a sulfonylurea as independent variables. For all comparisons a two-sided significance level of 0.05 was considered statistically significant. Data analysis was performed using the SPSS 20 software program, from IBM. RESULTS A total number of 954 patients were admitted in the period under study. From those, 200 admissions, corresponding to 196 patients (2 patients were admitted twice and 1 patient for three different times), were Heart, Lung and Vessels. 2014, Vol. 6 181 J.P.L. Nunes, et al. 182 Table 1 - Arithmetic mean and standard deviation (STD) for peak troponin I plasma values for patients with diabetes mellitus and acute myocardial infarction. Mean STD N Mean STD N SL DPP-4i 50.2 121.3 35 No DPP-4i 44.5 85.5 145 0.32 Insulin 39.2 108.4 56 No insulin 52.1 89.3 144 0.11 Metformin 45.8 97.3 93 No metformin 45.3 89.1 87 0.99 Sulfonylurea 42.4 77.7 52 No sulfonylurea 47.8 99.2 128 0.91 N = number; SL = significance level (Mann-Whitney U test); DPP-4i = dipeptidyl peptidase-4 inhibitors. selected as meeting the inclusion criteria. 127 patients were of the male sex and 69 were female. The mean age was 67.7±10.6 years. ST segment elevation myocardial infarction was present in 62 patients. Primary coronary angioplasty was carried out in 44 patients. The mean peak plasma cardiac troponin I values for the 200 admissions was 48.5±94.9 ng/ml. DPP-4 inhibitors (either vildagliptin or sitagliptin) were being taken on admission by 35 patients, insulin by 56 patients, metformin by 93 patients, and sulfonylureas by 52 patients. A small number of patients were taking other types of antidiabetic dugs. 31 patients were taking no antidiabetic therapy at admission. Nineteen patients were taking oral antidiabetic drugs, but it was impossible to establish which drugs were in use (either the patients did not recall the names of the drugs in use or the record was incomplete). As for the most commonly found specific anti-diabetic therapies the following mean values for peak plasma troponin I (in ng/ml) were seen: no anti-diabetic therapy, 68.6±93.1 (n=31); insulin alone 22.8±70.7 (n=37), metformin alone 48.9±98.5 (n= 31), sulfonylurea alone 106.9±166.5 (n=7), sulfonylurea plus metformin 27.9±38.2 (n=17); DPP4 inhibitors plus metformin 32.7±50.8 (n=14). The mean value for peak plasma tropo- nin for patients either taking or not taking DPP-4 inhibitors, insulin, metformin or sulfonylureas are shown in Table 1. Patients under DPP-4 inhibitors therapy had a mean peak cardiac troponin plasma level of 50.2±121.3 ng/ml (n=35), the corresponding value for insulin being 39.2±108.4 ng/ml (n=56), for metformin the value being 45.8±97.3 ng/ml (n=93) and for sulfonylureas, 42.4±77.7 ng/ml (n=52). None of these values was significantly different from the corresponding group of patients not taking each class of drug (Table 1). As Table 2 shows, almost all (32/35) patients under DPP-4 inhibitors were simultaneously using metformin, and many were using other anti-diabetic drugs. Linear regression analysis, taking peak plasma troponin I as dependent variable, and age, sex, plasma creatinine at admission, ST segment elevation and use of DPP-4 inhibitors as independent variables, yielded an overall significant result (ANOVA with F 5.1, significance level <0.01), however only the presence of ST segment elevation reached a significance level <0.05 (the presence of DPP-4 inhibitors had a significance level of 0.35). Table 2 shows some clinical characteristics of patients with acute myocardial infarction admitted while currently taking DPP4 inhibitors. Eight cases of elevated ST-segment infarction, including one case of intra-stent thrombosis, and a case with new left bundle branch block were seen. One Heart, Lung and Vessels. 2014, Vol. 6 Myocardial infarction and DPP-4 inhibitors Table 2 - Clinical characteristics of 35 patients with diabetes mellitus and acute myocardial infarction associated to the use of DDP-4 inhibitors. Plasma creatinine (mg/dL); troponin I (ng/mL). Age (years) Sex Peak Plasma Troponin I Creatinine ST segment Previous Previous Primary CABG/PCI AMI PCI Antidiabetic therapy 61 Male 0.182 0.9 58 Female 121.5 0.9 71 Male 0.174 1.3 61 Male 5.17 0.7 Vildagliptin/metformin 76 Female 4.8 1.1 Vildagliptin/metformin Sitagliptin/metformin New LBBB 1 1 Vildagliptin/metformin; insulin Sitagliptin/metformin 60 Male 1 1.5 Sitagliptin/metformin; other OAD 67 Female 38 1.1 Sitagliptin/metformin 73 Male 11.8 0.8 Vildagliptin/metformin; gliclazide; acarbose 72 Female 0.26 0.9 Vildagliptin/metformin; glimepiride 66 Male 64 1.1 Vildagliptin/metformin 66 Male 3.25 1 Sitagliptin/metformin 76 Male 18.28 0.9 PCI 1 Sitagliptin; gliclazide; pioglitazone 76 Female 3.77 0.6 CABG 1 Sitagliptin 54 Male 28.09 0.5 79 Female 75.8 0.9 PCI 1 62 Male 0.179 0.8 63 Male 0.47 1.1 82 Female 0.048 1 61 Female 0.36 0.9 67 Male 2.01 2.4 81 Male 5.69 0.8 CABG Sitagliptin; metformin; glibenclamide 78 Female 11.3 0.9 PCI Sitagliptin; glibenclamide/metformin 51 Male 77.7 0.7 78 Male 0.704 1 75 Male 26 0.8 Elevated 61 Male 691 1.2 Elevated*** 72 Male 0.141 1 60 Male 24 0.7 86 Male 0.309 1.1 CABG 69 Male 0.388 0.6 PCI Sitagliptin/metformin; gliclazide Elevated* 1 Vildagliptin/metformin Sitagliptin/metformin; gliclazide Sitagliptin/metformin; gliclazide PCI Sitagliptin; metformin; glibenclamide Sitagliptin; metformin ** CABG Vildagliptin/metformin; glimepiride Elevated 1 Vildagliptin/metformin Sitagliptin/metformin Sitagliptin/metformin; gliclazide PCI 1 **** 1 Elevated Vildagliptin/metformin; insulin Sitagliptin; gliclazide 1 1 Sitagliptin; metformin; glibenclamide Vildagliptin/metformin Sitagliptin/metformin; gliclazide 80 Female 176 2.4 66 Male 25.3 0.9 LBBB Sitagliptin/metformin 70 Male 147.8 1.1 Elevated 1 Sitagliptin/metformin; gliclazide 61 Male 45.1 0.9 Elevated 1 Sitagliptin/metformin; insulin 56 Male 144 1.0 Elevated 1 Vildagliptin/metformin; gliclazide Vildagliptin/metformin; gliclazide *Acute intra-stent thrombosis. **Severe aortic stenosis. ***Deceased. ****Thrombolysis. LBBB = left bundle branch block; PCI = coronary percutaneous intervention; CABG = coronary artery bypass graft surgery; AMI = acute myocardial infarction; OAD = oral anti-diabetic drug. Heart, Lung and Vessels. 2014, Vol. 6 183 J.P.L. Nunes, et al. 184 patient died. Peak plasma levels for cardiac troponin I varied in a relatively wide range, from minor elevations under 1 ng/ml, to values over 100 ng/ml (Table 2). DISCUSSION In the present study we describe a group of 35 diabetic patients with acute myocardial infarction under current DPP-4 inhibitors therapy. The vast majority of the patients were also taking metformin. Myocardial infarctions associated to the use of DPP-4 inhibitors have been shown to be very variable in terms of peak plasma cardiac troponin levels, the major parameter evaluated in the present study. Mean peak plasma troponin in myocardial infarctions associated to the use of DPP-4 inhibitors, however, was not significantly different from the corresponding value in patients under other forms of anti-diabetic therapy, the same happening to myocardial infarctions associated to the use of insulin, metformin or sulfonylureas. The treatment of type 2 diabetes mellitus has been associated to modest results in what concerns mortality and major cardiovascular disease, such as myocardial infarction and stroke. The clinical trials published in recent years, the Action to Control Cardiovascular Risk in Diabetes Study (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) and the Veterans Affairs Diabetes Study (VADT), all failed to show results of interest associated to intensive therapy, in what concerns cardiovascular disease. ACCORD has even shown an increased mortality rate associated to intensive anti-diabetic therapy. A meta-analysis (also including data from the United Kingdom Prospective Diabetes [UKPDS] Study), however, has shown that “intensive glucose control reduced the risk for some cardiovascular disease outcomes (such as nonfatal myocardial infarction), but did not reduce the risk for cardiovascular death or all-cause mortality, and increased the risk for severe hypoglycemia” (9), findings essentially corroborated by a similar study (10). The UKPDS 80 study did show favorable long-term effects of intensive therapy (“legacy effect”), however the cohort under study in UKPDS 80 was only a fraction of the original UKPDS group of patients. It has been speculated that “increases in levels of insulin, not glucose, may be etiologic in cardio-vascular disease risk” (11), and it has also been stated that “it can be argued that lowering HbA1c is not, in and by itself, a meaningful outcome” (12). It is in this setting that new classes of antidiabetic drugs have been created, among which the DPP-4 inhibitors have attracted a considerable degree of interest. Promising laboratory data concerning DPP-4 inhibition have been published, including improved endothelial function (13) and reduction of experimental infarct size in the rat (14). This group of drugs, which includes, among others, sitagliptin, vildagliptin and saxagliptin, are believed to act by inhibiting the enzyme dipeptidyl peptidase 4, which in turn degrades incretins such as GLP-1, hormones released postprandially, thereby increasing insulin and decreasing glucagon. DPP-4 inhibitors have been shown to decrease glycated hemoglobin with a neutral effect on body weight and a low risk for hypoglycemia (15). According to Jose and Inzucchi, DPP-4 substrates are extensive, and “DPP-4 is not specific for GLP-1 and therefore has the potential to mediate a wide range of pleiotropic effects (16).” Two major clinical trials on DPP-4 inhibitors have been published. Saxagliptin was compared to placebo in the Saxagliptin assessment of vascular outcomes recorded in Heart, Lung and Vessels. 2014, Vol. 6 Myocardial infarction and DPP-4 inhibitors patients with diabetes mellitus (SAVORTIMI 53) clinical trial (17). After a median follow-up of 2.1 years, the study concluded that “DPP-4 inhibition with saxagliptin did not increase or decrease the rate of ischemic events, though the rate of hospitalization for heart failure was increased” (17). In the Examination of cardiovascular outcomes with Alogliptin versus standard of care (EXAMINE) clinical trial, a total of 5380 patients with acute coronary syndrome were randomized to take alogliptin or placebo and followed for a median of 18 months (18). The authors concluded that the rates of major adverse cardiovascular events were not increased with alogliptin as compared with placebo (18). Meta-analyses and a pooled analysis (1921) failed to show an unfavorable cardiovascular profile for these drugs, however in the comparator arm there were not only data obtained with placebo but also with active comparators, thereby limiting the evaluation of DPP-4 inhibitors. In the present study, the vast majority of the patients with myocardial infarction associated to the use of DPP-4 inhibitors were also taking metformin. Linear regression analysis failed to indicate any significant influence on peak troponin I levels associated to the presence of DPP-4 inhibitors. From a theoretical standpoint, the presence of metformin could mask any possible effect of DPP-4 inhibitors on the patho-physiology of acute myocardial infarction. Peak plasma troponin I values in the 31 patients treated with metformin alone was 48.9±98.5 ng/ ml, a value which is very similar to the value for the 35 patients under DPP-4 therapy, 50.2±121.3 ng/ml. It is clearly difficult to evaluate, using the present data, any possible effects of DPP-4 inhibitors, by themselves, on myocardial infarction. We can reasonably suggest, however, that DPP-4 inhibitors therapy doesn’t seem to be associated to any increased importance of acute myocardial infarction in patients under metformin therapy, in what concerns peak plasma troponin I levels. In any case, both vildagliptin/metformin and sitagliptin/metformin have become extremely popular fixed drug associations, at least in Portugal, where they represented the second and third absolute top-selling drugs in the period January/March 2012 (22). Study limitations – The present study has significant limitations: it is a retrospective study; indication bias is probably present, in the sense that patients for whom doctors chose different types of anti-diabetic therapy were probably different from each other; for a considerable number of patients, it is known that they were under oral anti-diabetic therapy but the exact nature of that therapy is unknown; the duration of antidiabetic drug usage is also unknown; the small dimension of the sample limits the strength of conclusions; finally there are many factors influencing troponin levels in patients with myocardial infarction, reperfusion therapy being one of them (23). CONCLUSION In conclusion, we have described a group of 35 diabetic patients with acute myocardial infarction under current anti-diabetic therapy including a DPP-4 inhibitor. The vast majority of the patients were also taking metformin. We found no evidence that peak plasma troponin I was different between patient with acute myocardial infarction and use of DPP-4i, when compared to cases not under such therapy. REFERENCES 1. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993; 16: 434-44. 2. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with Heart, Lung and Vessels. 2014, Vol. 6 185 J.P.L. Nunes, et al. 186 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New Engl J Med. 1998; 339: 229-34. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010; 375: 2215-22. Miranda VP, Rodrigues MJ, Goncalves FR, Nunes JPL. Effects of hypoglycemic agents on mortality and major cardiovascular outcomes in patients with type 2 diabetes mellitus: a narrative review. Rev Port Cardiol 2009; 28: 1099-119. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356: 2457-71. Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol. 2006; 48: 2192-4. American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012; 35 (Suppl. 1): S11-63. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007; 28: 2525-38. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009; 151: 394-403. Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009; 52: 2288-98. Goodarzi MO, Psaty BM. Glucose lowering to control macrovascular disease in type 2 diabetes: treating the wrong surrogate end point? JAMA 2008; 300: 2051-3. Zannad F, Stough WG, Pocock SJ, Sleight P, Cushman WC, Cleland JG, et al. Diabetes clinical trials: helped or hindered by the current shift in regulatory requirements? Eur Heart J. 2012; 33: 1049-57. Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013; 77: 1337-44. Hausenloy DJ, Whittington HJ, Wynne AM, Begum SS, Theodorou L, Riksen N, et al. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucosedependent manner. Cardiovasc Diabetol. 2013; 12: 154. Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012; 344: 1369. Jose T, Inzucchi SE. Cardiovascular effects of the DPP-4 inhibitors. Diab Vasc Dis Res. 2012; 9: 109-16. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013; 369: 1317-26. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med. 2013; 369: 1327-35. Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011; 27 (Suppl. 3): 57-64. Patil HR, Al Badarin FJ, Al Shami HA, Bhatti SK, Lavie CJ, Bell DS, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol. 2012; 110: 826-33. White WB, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C, et al. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab. 2013; 15 668-73. INFARMED. Analise do Mercado de Medicamentos em Ambulatorio Março 2012. Lisbon, Portugal: Instituto da Farmacia e Medicamento; 2012. Katus HA, Remppis A, Scheffold T, Diederich KW, Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991; 67: 1360-7. Cite this article as: Nunes JPL, Rodrigues JD, Melão F. Acute myocardial infarction associated to DPP-4 inhibitors. Heart, Lung and Vessels. 2014; 6(3): 180-186. Source of Support: Nil. Disclosures: None declared. Heart, Lung and Vessels. 2014, Vol. 6 ORIGINAL ARTICLE Heart, Lung and Vessels. 2014; 6(3): 187-196 Agglutinins and cardiac surgery: a web based survey of cardiac anaesthetic practice; questions raised and possible solutions S. Shah1, H. Gilliland2, G. Benson3 1 Department of Anaesthesiology, Singapore General Hospital, Singapore; 2Cardiac Anaesthesia, Royal Victoria Hospital, Belfast, UK; 3Haematology and Blood Transfusion Services, Royal Victoria Hospital, Belfast, UK Heart, Lung and Vessels. 2014; 6(3): 187-196 ABSTRACT Introduction: Cardiac surgery involves cardiopulmonary bypass during which the core temperature is generally lowered to hypothermic levels. Patients presenting for cardiac surgery are sometimes reported to have cold or warm autoantibodies at the time of blood screening. It is known that cold agglutinins may cause potentially life-threatening haemolysis, intracoronary haemagglutination leading to inadequate cardioplegia distribution, thrombosis, embolism, ischaemia or infarction. The risk (if any) posed by warm autoantibodies is less clear. Because of the absence of hospital policies and of clear UK guidelines that explain how to manage such cases, we decided to conduct a web-based survey regarding standard anaesthesia practice in patients with both cold and warm autoantibodies presenting for cardiac surgery. Methods: We devised a short electronic survey asking for responses to 8 questions on cold auto-antibodies and 2 on warm auto-antibodies. This was sent to all members of the Association of Cardiothoracic Anaesthetists. Responses were collated and expressed as percentages. Free text responses were analysed for trend or reported verbatim. Results: The results of our survey demonstrate that there is no consensus on the appropriate management of such patients, with responses ranging from cancelling surgery to proceeding without additional precautions. Conclusions: In collaboration with haematologists and taking into account the available evidence, our institution has now developed a management strategy for cardiac patients with cold autoantibodies. Further studies will be required to determine the usefulness of our algorithm in daily practice. Keywords: cardiac, surgery, cold, warm, auto-antibody. INTRODUCTION Cardiac surgery involves the use of cardiopulmonary bypass (CPB) during which the core temperature is generally lowered to hypothermic levels. For this reason, the presence of cold agglutinins (CAs) is of particular significance for cardiac surgical patients. The main risk posed by CAs is of Corresponding author: Dr Shitalkumar Shah MB BS, DNB, FCARCSI, MRCA Singapore General Hospital, Outram road, Singapore 169608 e-mail: shital_s_shah@yahoo.com potentially life-threatening haemolysis. In addition, there is a risk of intracoronary haemagglutination leading to inadequate cardioplegia distribution, thrombosis, embolism, ischaemia or infarction (1-4). In our institution (Royal Victoria Hospital, Belfast, UK) cold agglutinins are not routinely tested preoperatively. Despite this, the laboratory not infrequently reports the presence of either CAs or “non-specific cold autoantibodies” (NSCAs) on the pre-operative group and screen test. For the majority of patients, this is a novel finding and there are no pointers to clinical cold agglutinin Heart, Lung and Vessels. 2014, Vol. 6 187 S. Shah, et al. 188 disease, such as acrocyanosis or laboratory evidence of haemolytic anaemia. The cardiac anaesthesiologist is thus presented with a problem: are these CAs or NSCAs likely to be of clinical significance and, if so, what steps should be taken to minimise the potential risk? In the absence of any hospital policy, the usual course of action in our institution has been to solicit haematological advice. Sometimes further tests are requested, delaying the proposed surgery, whilst on other occasions no specific action is prescribed. Adding to the general confusion, we have also encountered a small number of patients identified as having “warm” autoantibodies or agglutinins (WAs) at the time of preoperative screening for cardiac surgery. Because of the absence of any clear UK guidelines that explain how to manage such cases, we decided to conduct a web-based survey regarding standard anaesthesia practice in patients, presenting for cardiac surgery, with both cold and warm autoantibodies. METHODS We devised a short electronic survey consisting of 8 questions on CA/NSCAs and 2 questions on warm agglutinins in addition to one question about the identity of the responder’s institute. This national survey was approved by the committee of the Association of Cardiothoracic Anaesthetists (ACTA) and the survey questionnaire was sent electronically to all ACTA members over a 1 month period in July 2011. The 8 questions related to CA/NSCAs and the two relating to warm agglutinins are shown in Table 1 below. Responses were collated and the percentage positive response to each question calculated. Where free text was permitted in an answer, comments were analysed to identify trends or similarities. Where no trend ex- isted, the response was reported verbatim. Since the questionnaire asked about the general experience of ACTA members, responses were assumed to reflect both adult and paediatric practice. RESULTS We received a total of 40 completed questionnaires from 19 separate cardiac surgical institutes, 18 from the UK and 1 from North America. 6 responders preferred not to reveal name of their institution. The distribution of responses in shown in Table 2 below. These institutes carry either medium or high quantity work-load. In response to question 1, most cardiac anaesthesiologists (87.5%) said they had heard of cold agglutination syndrome, however, 10% stated that they had not. One responder declined to answer. In response to our second question, 37 out of 40 respondents (92.5%) had no protocol/ guideline in their institute. However, 3 responders from different institutes did have an established policy in their place of work. With regard to question 3, the majority of anaesthetists (60%) reported that they encountered less than 5 cases of non-specific cold autoantibodies per year in their practice. 27.5% said they never encountered this problem, 7.5% said they encountered between 5-10 cases per year and 2.5% stated that they experienced more than 10 cases each year in their practice. For the next set of questions, options were provided and multiple answers were allowed. Responding to question 4: 85% of responders would refer a patient with cold autoantibodies to a haematologist and 45% said they would alter the conduct of cardiopulmonary bypass. 25% would also order further investigations. By contrast, a small number of responders (10%) stated that they would not take any action if a patient Heart, Lung and Vessels. 2014, Vol. 6 Agglutinins and cardiac surgery 189 Table 1 - Questionnaire. Number Question 1 Are you aware of cold agglutination syndrome Y/N 2 Are you aware of any protocol in your hospital for patients with non specific cold or warm blood antibodies for cardiac surgery Y/N 3 How often in your cardiac practice do you encounter patient with non specific cold antibodies: • >10 times per year • 5-10 times per year • <5 times per year • never 4 What action do you take (in patients with cold autoantibodies). Tick any that apply: • None • Order further investigations • Refer to haematologist • Alter the conduct of cardiopulmonary bypass • Other (please specify below) 5 If you order further investigations which of the following do they include. Tick any that apply: • CBC and differential • blood film • antibody titre • thermal amplitude • liver function test • coombs test • other (please specify below) 6 Preoperatively, how would you manage raised cold antibody titres. Tick any that apply: • No treatment • steroids • high dose IgG • plasmapheresis 7 If you alter the conduct of CPB which of the following would you consider. Tick any that apply: • Will not alter CPB conduct • Off pump surgery • Normothermia • Warm blood cardioplegia • Warm plus cold crystalloid cardioplegia • Fibrillatory cross clamp • Other Do you consider myocardial temperature monitoring Y/N 8 If the proposed surgery necessitated deep hypothermic circulatory arrest, would you (single answer): • Proceed with additional precautions • Proceed after informed consent (no additional precautions) • Cancel the surgery If you answered additional precautions please specify below 9 How often in your cardiac practice do you encounter patients with warm antibodies 10 What action do you take in patients with warm antibodies. Tick any that apply: • Refer to haematologist • Alter the conduct of CPB • Order further investigations • No action Heart, Lung and Vessels. 2014, Vol. 6 S. Shah, et al. 190 Table 2 - Distribution of responses from UK hospitals . Institution No of respondents Freeman Hospital, Newcastle upon Tyne, UK 4 Papworth, Papworth, UK 4 Guys and St Thomas, London, UK 3 Bristol Royal, Bristol, UK 3 Royal Victoria Hospital, Belfast, UK 2 Royal Infirmary of Edinburgh, Edinburgh, UK 2 John Radcliffe Hospital, Oxford, UK 2 The Royal Sussex County Hospital, Brighton, UK 2 Royal Brompton Hospital, UK 2 GJNH, Glasgow, UK 1 Aberdeen Royal, Aberdeen, UK 1 The General Infirmary, Leeds, UK 1 James Cook University Hospital, UK 1 Hammersmith Hospital, London, UK 1 Barts and London, London, UK 1 Southampton University Hospital, UK 1 Wythenshaw Hospital, UK 1 Northern General hospital, Sheffield, UK 1 Toronto General Hospital, Canada 1 Anonymous 6 were found to have cold autoantibodies preoperatively. In response to question 5, when presented with a list of options, more anaesthesiologists appeared willing to request further investigations than the 25% who had stated that they would do so in answer to question 4. Antibody titre was the most popular choice (35%), followed by thermal amplitude, blood film and Coomb’s test (each 27.5%). 12.5% responded that they would order further investigations following the haematologist’s advice. One respondent stated that thermal amplitude testing was not available in their unit. In answer to question 6, the most popular choice was to refer the patient to a haematologist for preoperative management (37.5%), 27.5% would consider preoperative plasmapheresis, 20% would give preoperative steroids, 12.5% would consider high dose IgG and 7.5% said they would decide whether or not to provide preoperative treatment based on thermal amplitude results. A substantial number (30%) would not offer any treatment preoperatively. For the purposes of this question, we did not attempt to define “raised titres”. Next, we explored how the conduct of cardiopulmonary bypass should be altered in patients with high titre cold autoantibodies. The most popular option (70%) was to conduct the surgery at normothermia with 60% opting to also give warm blood cardioplegia. 47.5% also considered off-pump surgery (if feasible) and 20% would consider fibrillatory cross clamp as an option. Only 10% would include myocardial temperature monitoring in their perioperative strategy. 15% offered a free text answer that ranged from “would consult haematologist”, “would perform literature review” to “would cancel the surgery”. Question 8 presented responders with a difficult scenario. This time the options offered were mutually exclusive and the answers ranged from 10% who would proceed to deep hypothermic circulatory arrest without any additional precautions to 27.5% who would cancel the surgery altogether. The most popular option was to go ahead with surgery but to take additional precautions (42.5%). 20% declined to answer the question. When asked to comment on the additional precautions to be taken, most respondents would seek further advice before proceeding. 15% would ask for hae- Heart, Lung and Vessels. 2014, Vol. 6 Agglutinins and cardiac surgery matology advice whereas 17.5% preferred a multidisciplinary team approach. One respondent suggested conducting a literature review. Only 2 respondents appeared happy to devise a management plan without seeking further advice and both suggested using plasmapheresis preoperatively. Questions related to warm autoantibodies. Question 9 revealed that this problem appeared to be less common with 45% saying they never encountered it and another 40% quoting an incidence of less than 5 times in a year in their practice. We then asked on the action to be taken, five of those surveyed did not select any of the options. Of the offered options, the most popular was to talk to haematologists (chosen by 70%), 25% volunteered to alter the conduct of cardiopulmonary bypass and 12.5% would order further investigations. 17.5% would not alter any aspect of perioperative management. DISCUSSION This web-based survey demonstrates that there is considerable confusion with regard to the correct management of cardiac patients with cold or warm autoantibodies. In terms of cold autoantibodies, the survey shows that most cardiac anesthetists across the UK have the same experience as us. They see between 1 and 5 cases per year, they have no policy in place as to what to do with them, and the most popular and recurring option is to contact haematologists for advice and to do whatever they instruct. In terms of the actions taken, there is extreme variability with some anaesthetists willing to cancel surgery in any patient with CAs/ NSCAs and others prepared to go ahead with deep hypothermia even in the face of high antibody titres. Clearly, both cannot be right. It is possible that some patients are being placed at risk whilst others are hav- ing life-saving surgery postponed unnecessarily. With regard to warm autoantibodies, our survey showed that some cardiac anaesthesiologists never encountered the problem and that for some of those there was uncertainty about what to do next. Warm autoantibodies, although responsible for the majority of autoimmune haemolytic anaemias (AIHAs), are active at normal body temperature meaning that cardiac surgery presents little in the way of additional risk for these patients. There is therefore no benefit in altering the conduct of cardiopulmonary bypass. Nevertheless, the finding of warm autoantibodies should not simply be ignored. Prompt referral to a haematologist for further investigation and management is the appropriate course of action as suggested by 70% of our respondents. The remainder of this discussion will deal with cold autoantibodies since these present the greater potential risk for the cardiac surgical patient. Numerous case reports have discussed the investigation and management of cardiac patients with cold autoantibodies, however there is as yet no consensus on the best plan of action. There is an urgent need for institutional guidelines on the perioperative management of cardiac surgical patients with cold autoantibodies. Cold autoantibodies in cardiac surgery. Typical CA’s are IgM autoantibodies that react against I-antigens on the surface of erythrocytes. The cause of these CAs may be primary/idiopathic, or more commonly, secondary to an infective process (mycoplasma, infectious mononucleosis, other viral infections) or a lymphoproliferative disorder (5). The broader term “cold autoantibodies” describes a spectrum of cold reactive proteins ranging from the non-specific type, to the typical IgM CA. The finding of a cold autoantibody on routine cross matching may Heart, Lung and Vessels. 2014, Vol. 6 191 S. Shah, et al. 192 have a variety of implications depending on titre and thermal amplitude. Titre represents the highest dilution of the serum sample at which agglutination of red cells in the cold is still seen: the higher the titre, the greater the likelihood of clinically significant cold autoantibody activation. Low titre cold autoantibodies (<1:40) can be detected in virtually all normal subjects under appropriate conditions and are clinically insignificant. (6). Higher levels of autoantibody may predispose the patient to agglutination of blood in non-physiological situations (e.g. during induced hypothermia) whilst, most rarely, cold agglutinins can give rise to the cold agglutinin syndrome, a very rare type of autoimmune haemolytic anaemia (AIHA) with an estimated incidence of one case per million people per year (7). Amongst the cardiac surgical population the incidence of detectable cold autoantibody has been stated to be approximately 0.8% to 4% (8). Thermal amplitude is the temperature below which antibody activation occurs. Most patients have no symptoms at normothermia, but those with high titre and high thermal amplitude CAs can suffer haemagglutination at lower temperatures. If CAs are active at temperatures which also permit complement fixation, haemolysis may result. In the context of CPB, the initiation of rewarming can lead to catastrophic haemolysis (5). Clues to the presence of intraoperative agglutination/haemolysis include visible agglutination in the cardioplegia circuit, intracoronary thrombosis, inadequate delivery of cardioplegia and high line pressures in the cardiopulmonary bypass circuit (2, 9). The consequence of this process can be devastating with myocardial or cerebral infarction and multi-organ failure. Much of what is known about cold agglutinins and their consequences during cardiac surgery comes from case reports. Izzat et al. (4) report a case where agglutination of red blood cells occurred within a minute of initiation of antegrade cold blood cardioplegia at 10oC leading to embolization in the coronary microcirculation. When the agglutinates were noticed, a coronary sinus cannula was inserted through the right atrium and continuous retrograde cold crystalloid cardioplegia was infused. Agglutinates were noted to flush back from the coronary arteries into the aortic root and the patient did not show any signs of cardiac damage postoperatively. This suggests that agglutination per se may be remediable if prompt action is taken. Haemolysis on the other hand may be much less amenable to intervention. An interesting case report by Bracken et al (10) described cardiopulmonary bypass in a 67-year-old male patient with cold agglutinins that had gone undetected prior to surgery. During surgery, the red cells in the cardioplegia heat exchanger clumped and the patient was noted to have haemoglobinuria. On the evening of surgery, the patient developed a cold pulseless left leg and underwent a bedside revascularization procedure. He died on the second postoperative day of haemodynamic compromise. The authors commented that it is not clear that cold agglutinins were directly related to the terminal event. In contrast to the numerous case reports, a recent study by Barbara et al. (11) examined the incidence and consequences of cold agglutinins in the cardiac surgical population over an 8-year period. They reported only one case of haemolysis among 16 patients with either cold agglutinin disease or detectable CAs between 2002 and 2010. No serious harm resulted. Their findings might lead one to conclude that the presence of CAs is of little clinical significance, however it is worth noting that very few of these patients were exposed to any degree of hypothermia. In only one case was the core temperature allowed to drift below 34oC and 14 out of 16 procedures deliber- Heart, Lung and Vessels. 2014, Vol. 6 Agglutinins and cardiac surgery ately employed warm blood cardioplegia. The authors concluded that asymptomatic CAs can safely be managed at normothermia without the need for further testing. Nevertheless, some cardiac surgical procedures cannot be performed at normothermia, hence it is still important to be able to determine which cardiac surgical patients with CAs/NSCAs are at risk of agglutination/haemolysis during surgery. Identification of patients at risk. The determination of risk is informed by both clinical history and laboratory tests. Preoperative screening should include queries about symptoms/signs of cold agglutination including acrocyanosis, haemoglobinuria, jaundice, and pallor (12). Laboratory tests for haemolytic anaemia should also be used to determine whether or not the patient has the clinical syndrome of cold agglutination. Given the non-physiological conditions during cardiac surgery, patients without preoperative symptoms or signs of haemolytic anaemia may still be at risk intraoperatively. In the absence of evidence of autoimmune haemolytic anaemia, the most useful laboratory tests on which to base an assessment of risk are thermal amplitude and plasma titre (13). These are difficult tests to perform, as accuracy necessitates that the blood sample is maintained at 37°C until the serum has been removed. A titre of around 1:10 is typical in normal individuals and up to 1:40 may regarded as clinically insignificant. Haemolysis is rarely seen below titres of 1:1000 whilst individuals with the cold agglutinin syndrome typically have titres in excess of 1:10,000 (6). Antibody titre varies with temperature. The presence of high antibody titres at 4°C (1:10,000) and low antibody titres at 37°C (1:16) is typical. Yet, in some patients, antibody titres show a flatter thermal spectrum with a moderately high titre at 4°C (1:320) and a readily de- monstrable titre at 37°C (1:64) (14). In the typical profile of CA reactivity, profoundly hypothermic temperatures cause intense red cell agglutination, a process that reverses on rewarming. By contrast complement fixation is a warm-reactive process. Hence, complement-mediated haemolysis will only occur if the spectrum of temperatures that provoke agglutination overlaps that required for complement fixation. To summarise, the spectrum of risk posed by the presence of cold autoantibodies ranges from no risk at all to life-threatening red cell agglutination/haemolysis. In terms of cardiac surgery, the overall understanding is that patients with low-titre (<1:32), lowthermal amplitude CAs (<20°C) are not at particular risk of agglutination and may not warrant any alteration in surgical plan (8). Management. Management of patients deemed to be at risk of agglutination/haemolysis consists of preoperative strategies to reduce antibody titre or reactivity and intraoperative alterations to the conduct of surgery. Pre-operative strategies. Optimum pre-operative management for cardiac surgical patients with clinically significant CAs is still unclear. Administration of steroids, azathioprine and cyclophosphamide has not been shown to be of benefit (8). There have been numerous reports of the use of plasma exchange in patients with CAs (2, 15-21). Plasma exchange is a complex procedure that must be performed at normothermia and has several attendant risks including that of infection and of creating large volume shifts, which may be badly tolerated by cardiac patients. Since most CA IgM is intravascular, up to 80% of it may be removed by single 5-litre plasma exchange (22). Despite this, reports of the effectiveness of plasma exchange in the management of patients with CA have been mixed, with some suggesting success (18) while others have shown no benefit (20). Heart, Lung and Vessels. 2014, Vol. 6 193 S. Shah, et al. 194 IgG therapy is more costly but easier to administer than plasma exchange. A case report has shown that high-dose IgG administration just before cardiac surgery caused an 8-fold reduction in the titre of CAs. The mechanism of IgG’s action remains unclear. The authors speculate that the high dose IgG provides a protective coating over the I-antigens on erythrocytes and in this way prevents agglutination (23). Intraoperative alterations in the conduct of surgery. Several authors have reported successful outcomes from intraoperative measures taken to limit the haematologic and cardiac consequences of cold exposure in patients with CAs (24). The range of options for the intraoperative management of these patients is wide (1, 2, 4, 8, 9, 25-37). Reported strategies include: - Warm blood cardioplegia: antegrade and/ or retrograde - Warm ischaemic arrest - Warm crystalloid cardioplegia to flush coronaries followed by cold crystalloid cardioplegia Irrespective of the technique employed, it is essential to limit systemic cooling during CPB so as to maintain the systemic perfusion temperature above the thermal threshold of agglutinin activity. It is important not to forget simple measures such as the use of warming mattresses, heating of anaesthetic gases intravenous fluids and blood products. Similarly, the operating room temperature should be elevated. Management of intraoperative agglutination. Cold agglutinins may not be detected prior to surgery in some at risk patients (4, 9, 10, 26). First time detection of agglutination in the intraoperative period warrants immediate action to raise the core temperature to normothermia along with warm retrograde myocardial washout as described above. Further treatment should be directed towards ameliorating any resulting haemolysis or end-organ damage. Further progress in our institution as a result of this national survey. The results of our survey were shared with colleagues in both transfusion medicine and general haematology. We were able to explain the particular risks posed by cardiac surgery whilst our haematological colleagues could give advice on how to establish the significance or nonsignificance of cold autoantibodies. Working in collaboration with these experts, we developed local guidance (Royal Victoria Hospital, Belfast, UK) on managing cardiac surgical patients with preoperative cold autoantibodies (Figure 1). Briefly, the guidance recommends for all such patients to be investigated for symptoms/signs of haemolytic anaemia. In common with Barbara et al. (11), our guidance states that those patients with evidence of haemolytic anaemia must be referred to a haematologist for further management prior to surgery. Those patients without haemolytic anaemia may follow two pathways depending on the type of surgery. If the surgery is low-risk, it may be performed at modest hypothermia (34o C) with warm cardioplegia and without further testing. Surgery where significant hypothermia is necessary, or highly likely, requires thermal amplitude and titre testing preoperatively to determine the risk of agglutination/haemolysis. The early involvement of haematologists is essential in the perioperative management of this latter group of patients. CONCLUSION Cold or warm autoantibodies may be detected for the first time on the preoperative group and screen. Warm autoantibodies, although responsible for the majority of cases of autoimmune haemolytic anaemia, present little in the way of additional risk for cardiac surgical patients. Nevertheless, such patients should be referred to a haematolo- Heart, Lung and Vessels. 2014, Vol. 6 Agglutinins and cardiac surgery 195 Figure 1 - Algorithm for the management of cardiac surgical patients with cold autoantibodies. AIHA = autoimmune hemolytica; MCV = mean corpuscular volume; LDH = lactate dehydrogenase. gist for further investigation and management of their haemolytic anaemia. By contrast, cardiac surgery may pose additional risk for some patients with cold autoantibodies. Although many of these patients have a very low likelihood of agglutination/ haemolysis if hypothermia is employed, the management of patients with high titre, high thermal amplitude cold autoantibodies require meticulous planning before cardiac operations. The results of our survey demonstrate that the appropriate management of such patients remains unclear, with responses ranging from cancelling surgery to proceeding without additional precautions. Furthermore, the available literature yields no clear consensus on either the degree of risk posed by cooling or the antibody titre that precipitates a need for alteration in the conduct of surgery. Based on the results of this study, extensive literate review and collaboration with colleagues in haematology, we have developed and described a simple management algorithm for dealing with cardiac patients with cold autoantibodies. Further studies will be necessary to confirm the use of our algorithm in everyday practice. REFERENCES 1. Shahian DM, Wallach SR, Bern MM. Open heart surgery in patients with cold-reactive proteins. Surgical Clinics of North America 1985; 65: 315-322. 2. Park JV, Weiss CI. Cardiopulmonary bypass and myocardial protection: management problems in cardiac surgical patients with cold autoimmune disease. [Review]. Anesthesia & Analgesia 1988; 67: 75-78. 3. Menasche P, Subayi JB, Piwnica A. Retrograde coronary sinus cardioplegia for aortic valve operations: a clinical report on 500 patients. Annals of Thoracic Surgery 1990; 49: 556-563. 4. Izzat MB, Rajesh PB, Smith GH. Use of retrograde cold crystalloid cardioplegia in a patient with unexpected cold agglutination. Annals of Thoracic Surgery 1993; 56: 1395-1397. 5. Fischer GD, Claypoole V, Collard C. Increased pressures in the retrograde blood cardioplegia line: an unusual presen- Heart, Lung and Vessels. 2014, Vol. 6 S. Shah, et al. 196 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. tation of cold agglutinins during cardiopulmonary bypass. Anesth Analg. 1997; 84: 454-6. Rosse WF, Schrier SL, Mentzer WC, Landaw SA. Clinical features and treatment of autoimmune haemolytic anaemia, UpToDate, August 2013. Berensten S, Ulvestad E, Langholm R, Beiske K, HjorthHansen H, Ghanima W, et al. Primary chronic cold agglutinin disease. Haematologica. 2006; 91: 460-460. Agarwal SK, Ghosh PK, Gupta D. Cardiac surgery and coldreactive proteins. Annals of Thoracic Surgery. 1995; 60: 1143-50. Dake SB, Johnston MFM, Brueggeman P, Barner HB. Detection of cold haemagglutination in blood cardioplegia unit before systemic cooling of a patient with unsuspected cold agglutinin disease. Annals of Thoracic Surgery 1989; 47: 914-15. Bracken CA, Gurkowski MA, Naples JJ, Smith H, Steinmann A, Samuel J, et al. Case 6-1993. Cardiopulmonary bypass in two patients with previously undetected cold agglutinins. Journal of Cardiothoracic and Vascular Anesthesia 1993; 7: 743-749. Barbara DW, Mauermann WJ, Neal JR, Abel MD, Schaff HV, Winters JL. Cold agglutinins in patient undergoing cardiac surgery requiring cardiopulmonary bypass. Journal of Thoracic and Cardiovascular Surgery 2013; 146: 668-80. Hoffman JW, Gilbert TB, Hyder ML. Cold agglutinins complicating repair of aortic dissection using cardiopulmonary bypass and hypothermic circulatory arrest: case report and review. Perfusion 2002;17: 391-94. Should we worry about cold reactive antibodies in cardiac surgery? E-network forum, Californian Blood Bank Society. Accessed 11 Feb 2013. Packman CH, Leddy JP. Cryopathic hemolytic syndromes. In: Williams WJ, Butler E, Ersley AJ, Lichtman MA, eds. Hematolon. New York: McGraw Hill, 1990; 675-80. Klein HG, Faltz LL, McIntosh CL, Appelbaum FR, Deisseroth AB, Holland PV. Surgical hypothermia in a patient with a cold agglutinin. Management by plasma exchange. Transfusion 1980; 20: 354-357. Beebe DS, Bergen L, Palahniuk RJ. Anesthetic management of a patient with severe cold agglutinin hemolytic anemia utilizing forced air warming. Anesthesia & Analgesia 1993; 76: 1144-1146. Zoppi M, Oppliger R, Althaus U, Nydegger U. Reduction of plasma cold agglutinin titers by means of plasmapheresis to prepare a patient for coronary bypass surgery. Infusionsther Transfusionsmed. 1993; 20: 19-22. Jewski C Jr, Gault E, Briggs M, Silberstein L. Benefit of a 37 degree C extracorporeal circuit in plasma exchange therapy for selected cases with cold agglutinin disease. Journal of Clinical Apheresis 1988; 4: 13-17. Mastrogiovanni G, Masiello P, Iesu S, Senese I, Di Benedetto G. Management of cold agglutinemia with intermittent warm blood cardioplegia and normothermia. Annals of Thoracic Surgery 1996; 62: 317. Rodenhuis S, Maas A, Hazenberg CA, Das PC, Nieweg HO. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. Inefficacy of plasma exchange in cold agglutinin hemolytic anemia-a case study. Vox Sang. 1985; 49: 20-25. Shuma KH, Rock GA. Therapeutic plasma exchange. N Engl J Med. 1984; 310: 762-771. Powles R, Smith C, Kohn J, Hamilton Fairley G. Method of removing abnormal protein rapidly from patients with malignant paraproteinaemias. Br Med J. 1971; 3: 664-7. Kanemitsu S, Onoda K, Yamamoto K, Shimpo H. Simple preoperative management for cold agglutinins before cardiac surgery. J Thorac Cardiovasc Surg. 2010; 140: 73-74. Shulman IA, Petz LD. Red cell compatibility testing: Clinical significance and laboratory methods. In: Petz LD, Swisher SN, Kleinman S, Spence RK, Strauss RG, editors. Clinical Practice of Transfusion Medicine. New York: Churchill Livingstone, 1996: 199-244. Bedrosian CL, Simel DL. Cold hemagglutinin disease in the operating room. South Med J. 1987; 80: 466-471. Diaz JH, Cooper ES, Ochsner JL. Cardiac surgery in patients with cold autoimmune diseases. Anesth Analg. 1984; 63: 349-352. Holman WL, Smith SH, Edwards R, Huang ST. Agglutination of blood cardioplegia by cold-reacting autoantibodies. Ann Thorac Surg. 1991; 51: 833-835. Ko W, Isom OW. Cardiopulmonary bypass procedures in patients with cold-reactive hemagglutination. A case report and a literature review. J J Cardiovasc Surg (Torino). 1996; 37: 623-626. Paccagnella A, Simini G, Nieri A, Da Col U, Frugoni C, Valfre C. Cardiopulmonary bypass and cold agglutinin. J Thorac Cardiovasc Surg. 1988; 95: 543. Aoki A, Kay GL, Zubiate P, Ruggio J, Kay JH. Cardiac operation without hypothermia for the patient with cold agglutinin. Chest 1993; 104: 1627-1629. Hearnsberger J, Ziomek S, Tobler G, Maxson T, VanDevanter S, Harrell JE, Jr. Management of cold agglutinemia with warm heart surgical intervention: a case report. J Thorac Cardiovasc Surg. 1993; 106: 756-757. Lichtenstein SV, Salerno TA, Slutsky AS. Pro: warm continuous cardioplegia is preferable to intermittent hypothermic cardioplegia for myocardial protection during cardiopulmonary bypass. J Cardiothorac Anesth. 1990; 4: 279-281. Salerno TA, Houck JP, Barrozo CA, Panos A, Christakis GT, Abel JG, et al. Retrograde continuous warm blood cardioplegia: a new concept in myocardial protection. Ann Thorac Surg. 1991; 51: 245-247. Lichtenstein SV, Ashe KA, el Dalati H, Cusimano RJ, Panos A, Slutsky AS. Warm heart surgery. J Thorac Cardiovasc Surg. 1991; 101: 269-274. Roe BB. Warm blood cardioplegia: back to square one. Ann Thorac Surg. 1993; 55: 330-331. Muehrcke DD, Torchiana DF. Warm heart surgery in patients with cold autoimmune disorders. Ann Thorac Surg. 1993; 55: 532-533. Gokhale AG, Suhasini T, Saraswati V, Chandrasekhar N, Rajagopal P. Cold agglutinins and warm heart surgery. J Thorac Cardiovasc Surg. 1993; 105: 557. Cite this article as: Shah S, Gilliland H, Benson G. Agglutinins and cardiac surgery: a web based survey of cardiac anaesthetic practice; questions raised and possible solutions. Heart, Lung and Vessels. 2014; 6(3): 187-196. Source of Support: Nil. Disclosures: None declared. Acknowledgement: We would like to thank Dr Kathryn Maguire (Consultant Haematologist, Transfusion Services, Northern Ireland Blood Transfusion Services) for her assistance in reviewing this paper and developing the management algorithm. Heart, Lung and Vessels. 2014, Vol. 6 ORIGINAL ARTICLE Heart, Lung and Vessels. 2014; 6(3): 197-203 Direct comparison between cerebral oximetry by INVOSTM and EQUANOXTM during cardiac surgery: a pilot study A. Pisano1, N. Galdieri1, T.P. Iovino1, M. Angelone1, A. Corcione2 1 Cardiac Anesthesia and Intensive Care Unit, “Monaldi” Hospital A.O.R.N. “Dei Colli”, Naples, Italy; Anesthesia and Postoperative Intensive Care Unit, “Monaldi” Hospital A.O.R.N. “Dei Colli”, Naples, Italy 2 Heart, Lung and Vessels. 2014; 6(3): 197-203 ABSTRACT Introduction: Several near-infrared spectroscopy oximeters are commercially available for clinical use, with lack of standardization among them. Accordingly, cerebral oxygen saturation thresholds for hypoxia/ischemia identified in studies conducted with INVOSTM models do not necessarily apply to other devices. In this study, the measurements made with both INVOSTM and EQUANOXTM oximeters on the forehead of 10 patients during conventional cardiac surgery are directly compared, in order to evaluate the interchangeability of these two devices in clinical practice. Methods: Cerebral oxygen saturation measurements were collected from both INVOSTM 5100C and EQUANOXTM 7600 before anesthetic induction (baseline), two minutes after tracheal intubation, at cardiopulmonary bypass onset/offset, at aortic cross-clamping/unclamping, at the end of surgery and whenever at least one of the two devices measured a reduction in cerebral oxygen saturation equal to or greater than 20% of the baseline value. Bland-Altman analysis was used to compare the bias and limits of agreement between the two devices. Results: A total of 140 paired measurements were recorded. The mean bias between INVOSTM and EQUANOXTM was -5.1%, and limits of agreement were ±16.37%. Considering the values as percent of baseline, the mean bias was -1.43% and limits of agreement were ±16.47. A proportional bias was observed for both absolute values and changes from baseline. Conclusions: INVOSTM and EQUANOXTM do not seem to be interchangeable in measuring both absolute values and dynamic changes of cerebral oxygen saturation during cardiac surgery. Large investigations, with appropriate design, are needed in order to identify any device-specific threshold. Keywords: near-infrared spectroscopy, cerebral oximetry, cardiac surgery. INTRODUCTION In recent years, near-infrared spectroscopy (NIRS) is increasingly used to monitor regional cerebral oxygen saturation (rSO2) during cardiac surgery (1-4). In fact, neurologic injury is still a common complication Corresponding author: Antonio Pisano, MD Via Cupa della Torretta, 20 80070 Bacoli (NA), Italy e-mail: antoniopisanoMD@libero.it after cardiac surgery, with rates of postoperative neurocognitive decline (PONCD) and stroke of up to 50% and 1-3%, respectively (5). Moreover, stroke after cardiac surgery results in a 10-fold increase in mortality and in a 3-fold increase in hospital stay (6). In an attempt to reduce these potentially disastrous complications, NIRS has been therefore advocated as a routine monitor to prevent or minimize brain injury by detecting cerebral oxygen supply-demand imbalances (7-9). Actually, several experimental Heart, Lung and Vessels. 2014, Vol. 6 197 A. Pisano, et al. 198 data are gradually accumulating to show that both preoperative values and intraoperative changes of rSO2 can predict important complications and long-term outcomes after cardiac surgery, including stroke (10), delirium (11), neurocognitive decline (12), organ dysfunction (13, 14), length of hospital stay (12, 13, 15) and mortality (14). Nevertheless, not everyone considers these evidences sufficient to justify the routine use of NIRS monitoring in cardiac surgery (4, 16), that remains debated (7, 17) and poorly defined, with little consensus for its appropriate use (3). Probably, one of the reasons for this is the poor agreement among different devices (3, 7, 17-19), that has been also confirmed by two recent investigations (2, 20). Currently, several NIRS devices are commercially available for clinical use, with lack of standardization among them (2, 16, 19, 21). In fact, although the various models are mostly based on spatial resolution spectroscopy (16, 22), they differ in numerous important aspects related to the acquisition of their cerebral oxygen saturation measurements, including the algorithms adopted, the type of light source, the wavelengths of light emitted and the distance between the various light emitters and detectors (19). This makes comparisons between clinical studies using different devices difficult and, therefore, rSO2 thresholds for the development of hypoxia/ischemia remain elusive (23, 24). Particularly, since the majority of clinical data currently available have been generated using various INVOSTM devices (the first to be approved by the U.S.A. Food and Drug Administration) (16), it is not clear whether the thresholds identified in studies conducted with these models (11, 12-14, 25) may also apply to devices from other companies. In the present study, for the first time far as the authors know, the measurements made by both INVOSTM and EQUANOXTM NIRS oximeters on the forehead of adult patients during different moments of cardiac surgical procedures are directly compared, with the objectives of evaluating both the feasibility of such simultaneous measurements and the interchangeability of the two devices in clinical practice. METHODS The study protocol was approved by the local Ethical Committee. After informed consent, 10 patients (6 males, 4 females), mean age 65.1 ± 15.84, scheduled for conventional cardiac surgery with or without cardiopulmonary bypass (CPB) were enrolled in the study (Table 1). Two different NIRS monitors were applied to patients: the 2-wavelength INVOSTM 5100C (Somanetics, Troy, MI) and the 3-wavelength EQUANOXTM 7600 (Nonin Medical, Inc, Plymouth, MN). After rubbing and cleaning the skin with an alcohol swab, 2 sensors (one left and one right) Adult SomaSensor SAFB-SM (Covidien, Mansfield, MA) and 2 sensors (one left and one right) EQUANOXTM ADVANCETM Sensor-Adult model 8004 CA (Nonin Medical, Inc, Plymouth, MN) were placed over the forehead of the patients, as close as possible, being careful not to overlap light emitters and detectors. INVOSTM sensors were placed lower than EQUANOXTM ones in five patients, and higher than EQUANOXTM ones in the other five (Figure 1). All sensors where then connected to the respective devices via their proprietary cables. Regional cerebral oxygen saturation (rSO2) measurements were collected before anesthetic induction with patients breathing ambient air (baseline values), two minutes after tracheal intubation, two minutes after CPB onset, at aortic cross-clamping, at aortic unclamping, at CPB offset (when appli- Heart, Lung and Vessels. 2014, Vol. 6 Direct comparison between INVOSTM and EQUANOXTM in cardiac surgery Table 1 - Age, sex, type of surgery, number of measurements recorded (from each device) and number of desaturations ≥20% from baseline (displayed by one or both of the two devices) of the patients investigated. Patient N. Sex Age (years) Type of surgery N. of N. of desaturations ≥20% from baseline measurements INVOSTM EQUANOXTM Both (L+R) 1 M 63 AVR 14 0 0 0 2 M 60 CABG 20 5 3 3 3 F 79 OPCAB 12 5 0 0 4 M 79 MVR+ CABG 20 6 0 0 5 M 75 CABG 14 0 0 0 6 M 44 AVR 14 0 0 0 7 M 75 MVR 14 0 0 0 8 F 70 OPCAB 6 0 0 0 9 F 74 OPCAB 12 4 0 0 10 F 32 MVR 14 0 0 0 M = male; F = female; L = left; R = right; AVR = aortic valve replacement; CABG = coronary artery bypass graft; OPCAB = off-pump coronary artery bypass; MVR = mitral valve replacement. cable), at the end of surgery and whenever at least one of the two devices measured a bilateral or monolateral reduction in cerebral oxygen saturation equal to or greater than 20% of the baseline value. Each measurement, as well as an absolute value, was also recorded as a percentage of the respective baseline value according to the formula: % of baseline=absolute valuex100/baseline value. In all patients, whilst the EQUANOXTM monitor seemed not significantly influenced by the presence of the operating INVOSTM sensors, no rSO2 values were displayed (and a “poor signal quality” error message appeared) on the INVOSTM Figure 1 - Relative positioning of NIRS sensors on the forehead of patients 1, 3, 5, 7 and 9 (panel A) and 2, 4, 6, 8 and 10 (panel B). NIRS = near-infrared spectroscopy. Heart, Lung and Vessels. 2014, Vol. 6 199 A. Pisano, et al. 200 oximeter when the EQUANOXTM device was switched on. For this reason, it was not possible to record the rSO2 values from the two devices simultaneously. Therefore, immediately after collecting INVOSTM data, it was turned off and, simultaneously, the EQUANOXTM device was turned on and its data were recorded. Bland-Altman analysis was used to compare the bias and limits of agreement (bias ± standard deviation x 1.96) between the two devices. Moreover, linear regression was applied to the Bland-Altman plots in order to test the presence of a proportional bias. IBM SPSS Statistics software v 19.0 (IBM, Armonk, New York) was used for statistical analysis. A 2-tailed value of p<0.05 was considered significant. RESULTS A total of 140 measurements (70 left, 70 right) were collected from both devices in the 10 patients (Table 1). The mean bias between INVOSTM and EQUANOXTM was -5.1%, and limits of agreement were ±16.37%. (Figure 2A) The Bland-Altman plot showed the presence of a statistically significant proportional bias (n=140; R=0.541; R2=0.293; p=0.000). When considering the dynamic changes of rSO2 (expressed as percent of baseline) showed by the two devices (120 measurements), the mean bias was -1.43% and limits of agreement were ±16.47 (Figure 2B). Also in this case, there was a significant proportional bias (n=120; R=0.680; R2=0.462; p = 0.000). Interestingly, of the 20 total episodes (considering each individual sensor) of significant (or “threshold”) cerebral desaturation (i.e., a reduction equal to or greater than 20% from baseline) (25, 26) reported by INVOSTM in four patients, mostly during heart displacement for coronary artery exposure or during episodes of hypotension over CPB, only 3 (in one patient) were also reported by EQUANOXTM. No other “threshold” desaturation was reported by EQUANOXTM (Table 1). All significant desaturations were prompt- Figure 2 - Bland-Altman analysis between absolute values (panel A) and changes from baseline (panel B) of rSO2 measured by INVOSTM and EQUANOXTM in the 10 patients. Heart, Lung and Vessels. 2014, Vol. 6 Direct comparison between INVOSTM and EQUANOXTM in cardiac surgery ly corrected with conventional strategies (such as administer fluids or vasopressors or raise pump flow) (13) and no patients had complications. DISCUSSION The results of this investigation suggest a clinically important difference between INVOSTM and EQUANOXTM in measuring cerebral oxygen saturation as well as its variations during cardiac surgery, probably due to some of the characteristics in which they differ (such as the number of light emitters and detectors, the different distance between them and, consequently, a different tissue penetration of light, the number of wavelengths adopted, and a different builtin proprietary algorithm to assess oxygen saturation) (20). In particular, in our series the bias between INVOSTM and EQUANOXTM in measuring absolute values of rSO2 showed a moderate correlation with the mean values from the two devices, with a tendency of INVOSTM to underestimation (or a tendency of EQUANOXTM to overestimation) for the lower values. Of course, it is rather difficult to determine which of the two devices provide the “more true” absolute values. Most importantly, the limits of agreement were very wide. Accordingly, the two devices do not seem to be interchangeable in routine clinical practice. Therefore, the results of previous investigations that identified threshold absolute values of rSO2 able to predict outcomes such as postoperative delirium (11) and mortality (14) using INVOSTM may not to be applicable to EQUANOXTM. While these results are not particularly surprising, given that a poor reliability of absolute values of rSO2 measured by INVOSTM had already been showed (27, 28), the similar differences observed also in changes from baseline given by the two devices require caution in interpreting trends given by EQUANOXTM according to thresholds previously identified in studies using INVOSTM (1, 12-14, 25, 26). In fact, although the mean inter-device bias for dynamic changes of rSO2 was lower than that for the absolute values, the limits of agreement were, also in this case, wide. Moreover, an even stronger proportional bias was observed, meaning that the two devices may display a different magnitude of desaturation in similar clinical situations. Accordingly, we observed much more “threshold” desaturations from INVOSTM than from EQUANOXTM, although the number of episodes is not sufficient for confident statistical analysis. Of course, these results do not exclude that both devices may adequately describe the variations in cerebral oxygen supplydemand balance, although device-specific thresholds are probably needed to interpret correctly these variations in clinical practice. Our findings are consistent with the results of other recent investigations. In fact, even if this is the first report of a direct comparison between INVOSTM and EQUANOXTM measurements at cerebral level and in a clinical context, the agreement between the two devices has been previously evaluated, both directly (2, 20) and indirectly (2, 19, 29), in healthy volunteers. Davie et al. (19) reported variable sensitivity to extracranial tissue contamination among INVOSTM, EQUANOXTM, and FORE-SIGHT® (CAS Medical Systems, Inc, Brandford, CT) devices. This may partly explain the interdevice differences in absolute values of rSO2, but should not significantly affect the variations from baseline. However, Fellahi et al. (20) and Hyttel-Sorensen et al. (29) demonstrated that INVOSTM and EQUANOXTM, positioned simultaneously on calves or one at the time on forearms of healthy Heart, Lung and Vessels. 2014, Vol. 6 201 A. Pisano, et al. 202 volunteers, respectively, are not comparable in measuring both absolute values and dynamic changes of peripheral rSO2 after vascular occlusion tests. Finally, Bickler et al. (2) evaluated the performance of five commercially available cerebral oximeters (including INVOSTM and EQUANOXTM), applied two at the time per subject in 23 adult healthy volunteers, and found a large variation in reading bias (calculated as the difference between the instrument’s reading with the weighted saturation of venous and arterial blood) between subjects, especially during hypoxia. Similar differences were also found when comparing INVOSTM with other devices (18, 30). This study has several limitations, the most important being the small number of patients enrolled. However, the number of paired measurements, that can be considered independent among themselves, allows us to give some significance to our findings. One of the reasons why we limited our observations to a few patients is the convinction, gained during the collection of these initial data, that different study designs may be preferable in order to investigate the differences among the various devices. In fact, direct comparison of two NIRS devices seems to be somewhat difficult, since the sampled areas, though close, are not the same and interferences between the two sensors can not be excluded (20, 30). Regarding the latter, we report for the first time an important interference, due to which the paired measurements were not simultaneous but spaced each other by a few seconds, that is another important limitation of the present study. Maybe, in other investigations not reporting such interference, the distances between sensors were greater: in fact, Fellahi et al. (20) placed the sensors on calves, while Bickler et al. (2) applied only two sensors (one for each device) on the forehead, instead of four as in this report. A plausible explanation for IN- VOSTM but not EQUANOXTM being markedly disturbed by the other device is the use of three wavelengths (730, 810 and 880 nm) by EQUANOXTM and two wavelengths by INVOSTM, in particular two of the three used by EQUANOXTM (730 and 810 nm). CONCLUSION The present study suggests that INVOSTM and EQUANOXTM are not interchangeable in measuring both absolute values and dynamic changes of cerebral rSO2 during cardiac surgery. Accordingly, device-specific thresholds are probably needed to guide interventions aimed to prevent postoperative brain injury as well as other adverse outcomes. Since direct comparison of NIRS devices in such clinical context seems to be of poor feasibility and difficult interpretation, large investigations on each device are needed in order to identify any of such specific thresholds and to allow a more extensive and better-defined use of this promising technology. REFERENCES 1. Deschamps A, Lambert J, Couture P, Rochon A, Lebon JS, Ayoub C, et al. Reversal of decreases in cerebral saturation in high-risk cardiac surgery. J Cardiothorac Vasc Anesth 2013; 27: 1260-6. 2. Bickler PE, Feiner JR, Rollins MD. Factors affecting the performance of 5 cerebral oximeters during hypoxia in healthy volunteers. Anesth Analg 2013; 117: 813-23. 3. Zacharias DG, Lilly K, Shaw CL, Pirundini P, Rizzo RJ, Body SC, et al. Survey of the clinical assessment and utility of near-infrared cerebral oximetry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014; 28: 308-16. 4. Zheng F, Sheinberg R, Yee MS, Ono M, Zheng Y, Hogue CW. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg 2013; 116: 663-76. 5. Newman M, Mathew J, Grocott H, Mackensen GB, Monk T, Welsh-Bohmer KA, et al. Central nervous system injury associated with cardiac surgery. Lancet 2006;368:694-703. 6. Gottesman RF, Sherman PM, Grega MA, Yousem DM, Borowicz LM Jr, Selnes OA, et al. Watershed strokes after cardiac surgery: Diagnosis, etiology, and outcome. Stroke 2006; 37: 2306-11. 7. Vernick WJ, Gutsche JT. Pro: cerebral oximetry should be a routine monitor during cardiac surgery. J Cardiothorac Vasc Anesth 2013; 27: 385-9. Heart, Lung and Vessels. 2014, Vol. 6 Direct comparison between INVOSTM and EQUANOXTM in cardiac surgery 8. Fischer GW. Recent advances in application of cerebral oximetry in adult cardiovascular surgery. Semin Cardiothorac Vasc Anesth 2008; 12: 60-9. 9. Fedorow C, Grocott HP. Cerebral monitoring to optimize outcomes after cardiac surgery. Curr Opin Anaesthesiol 2010; 23: 89-94. 10. Goldman S, Sutter F, Ferdinand F, Trace C. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. Heart Surg Forum. 2004; 7: E376-81. 11. Schoen J, Meyerrose J, Paarmann H, Heringlake M, Hueppe M, Berger KU. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care 2011; 15: 218. 12. Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM 3rd, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg 2009; 87:36-44. 13. Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007; 104: 51-8. 14. Heringlake M, Garbers C, Käbler JH, Anderson I, Heinze H, Schön J, et al. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology. 2011; 114: 58-69. 15. Apostolidou I, Morrissette G, Sarwar MF, Konia MR, Kshettry VR, Wahr JA, et al. Cerebral oximetry during cardiac surgery: the association between cerebral oxygen saturation and perioperative patient variables. J Cardiothorac Vasc Anesth. 2012; 26: 1015-21. 16. Ghosh A, Elwell C, Smith M. Review article: cerebral nearinfrared spectroscopy in adults: a work in progress. Anesth Analg 2012; 115: 1373-83. 17. Gregory A, Kohl BA. Con: near-infrared spectroscopy has not proven its clinical utility as a standard monitor in cardiac surgery. J Cardiothorac Vasc Anesth 2013; 27: 390-4. 18. Dullenkopf A, Frey B, Baenziger O, Gerber A, Weiss M. Measurement of cerebral oxygenation state in anaesthetized children using the INVOS 5100 cerebral oximeter. Paediatr Anaesth 2003; 13: 384-91. 19. Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: A comparison 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. of three cerebral oximetry technologies. Anesthesiology 2012; 116: 834 -40. Fellahi JL, Butin G, Fischer MO, Zamparini G, Gérard JL, Hanouz JL. Dynamic evaluation of near-infrared peripheral oximetry in healthy volunteers: a comparison between INVOS and EQUANOX. J Crit Care 2013; 28: 881. Highton D, Elwell C, Smith M. Noninvasive cerebral oximetry: is there light at the end of the tunnel? Curr Opin Anaesthesiol 2010; 23: 576-81. Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009; 103: (Suppl. 1): 3-13. Smith M, Elwell C. Near-infrared spectroscopy: shedding light on the injured brain. Anesth Analg 2009; 108: 105557. Smith M. Shedding light on the adult brain: a review of the clinical applications of near-infrared spectroscopy. Philos Trans A Math Phys Eng Sci. 2011;369:4452-69. Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007; 11: 274-81. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000; 93: 964-70. Dullenkopf A, Baulig W, Weiss M, Schmid ER. Cerebral near-infrared spectroscopy in adult patients after cardiac surgery is not useful for monitoring absolute values but may reflect trends in venous oxygenation under clinical conditions. J Cardiothorac Vasc Anesth. 2007; 21: 535-9. Leyvi G, Bello R, Wasnick JD, Plestis K. Assessment of cerebral oxygen balance during deep hypothermic circulatory arrest by continuous jugular bulb venous saturation and near-infrared spectroscopyJ Cardiothorac Vasc Anesth. 2006; 20: 826-33. Hyttel-Sorensen S, Hessel TW, Greisen G. Peripheral tissue oximetry: comparing three commercial near-infrared spectroscopy oximeters on the forearm J Clin Monit Comput. 2014; 28: 149-55. Moerman A, Vandenplas G, Bové T, Wouters PF, De Hert SG. Relation between mixed venous oxygen saturation and cerebral oxygen saturation measured by absolute and relative near-infrared spectroscopy during off-pump coronary artery bypass grafting. Br J Anaesth 2013; 110: 258-65. Cite this article as: Pisano A, Galdieri N, Iovino TP, Angelone M, Corcione A. Direct comparison between cerebral oximetry by INVOSTM and EQUANOXTM during cardiac surgery: a pilot study. Heart, Lung and Vessels. 2014; 6(3): 197-203. Source of Support: Nil. Disclosures: Dr. Galdieri and Dr. Pisano have lectured in a course entitled “INVOS monitoring in cardiac and vascular surgery”. Dr. Galdieri received a grant from Covidien for this. Heart, Lung and Vessels. 2014, Vol. 6 203 CASE REPORT Heart, Lung and Vessels. 2014; 6(3): 204-207 204 Life threatening tension pneumothorax during cardiac surgery. A case report A. Jain, D. Arora, R. Juneja, Y. Mehta, N. Trehan Medanta Institute of Critical Care and Anesthesiology, Medanta The Medicity, Gurgaon, Haryana Heart, Lung and Vessels. 2014; 6(3): 204-207 ABSTRACT Tension pneumothorax is a life threatening condition that occurs when the intrapleural pressure exceeds atmospheric pressure. It requires prompt diagnosis and immediate treatment. Tension pneumothorax developing postoperatively after cardiac surgery is not uncommon but occurrence in the operating room during cardiac surgery is rare. We report a case of tension pneumothorax intraoperatively during off pump coronary artery bypass grafting. Keywords: pneumothorax, cardiac surgery, hypotension. INTRODUCTION CASE REPORT Tension pneumothorax occurs due to a one way communication between lung parenchyma and the pleural cavity leading to air entrapment in the pleural cavity with each inspiration with inability to release it during expiration. It requires prompt diagnosis and immediate treatment or it may lead to respiratory failure and cardiovascular collapse (1, 2). Tension pneumothorax developing postoperatively after cardiac surgery is not uncommon but occurrence intraoperatively during cardiac surgery is rare and not yet reported in the English literature. We report a case of tension pneumothorax occurring intraoperatively during off pump coronary artery bypass grafting (OPCAB). A 62 years, 60 kg male with coronary artery disease, past history of smoking (60 packyears), history of dyspnoea and chest pain on exertion NYHA grade II-III since one year was electively admitted for OPCAB. There was no history of chronic cough, recurrent respiratory tract infections, previous hospitalization, use of beta2 agonist or steroids, or history suggestive of chronic obstructive pulmonary disease (COPD), occupational lung disease or tuberculosis. General physical and systemic examination was within normal limits. Preoperative haematological investigations and pulmonary function test (PFT) were within normal limits. Chest radiograph revealed emphysematous changes. Transthoracic echocardiography showed no regional wall motion abnormality (RWMA) with normal valvular and left ventricular (LV) function. Induction of general anesthesia was un- Corresponding author: Dr. Ashish Jain DNB Anesthesia Medanta- The Medicity Sector 38, Gurgaon, Haryana, INDIA e-mail: drashishjain07@rediffmail.com Heart, Lung and Vessels. 2014, Vol. 6 Tension pneumothorax during cardiac surgery eventful and anesthesia was induced with fentanyl sulphate, midazolam, thiopentone sodium and maintained with isoflurane and air oxygen mixture. Orotracheal intubation was easy, facilitated with vecuronium bromide and without airway trauma. Intermittent vecuronium bromide and fentanyl sulphate were used intravenously in standard doses. Pulmonary artery catheter introducer sheath and a triple lumen central venous catheter were inserted in the right internal jugular vein under ultrasound guidance in the first attempt without any complication. A pulmonary artery catheter was then floated through the sheath. During OPCAB the left pleura was opened whilst harvesting the left internal mammary artery while the right pleura remained intact. The left lung was observed to be hyperinflated without any evidence of bullae. The patient was mechanically ventilated on volume control ventilation mode under low flow anesthesia at 1.0 litre/minute with a tidal volume (TV) of 8 ml/kg, respiratory rate of 14/min and I:E ratio of 1:2.5 without application of PEEP, achieving a peak inspiratory pressure (PIP) of 18 cm of H2O. During coronary artery grafting the tidal volume was decreased to 5 ml/kg and respiratory rate increased to 20/min because the left lung was obscuring the surgical field. Post induction arterial blood gas analysis (ABG) showed PaO2 of 80 mm Hg on FiO2 of 0.6 with rest of the values in normal range. Intraoperative endotracheal suctioning revealed excessive tracheobronchial secretions, simultaneously FiO2 was raised to 1.0 and subsequent ABG remained within normal limits. Major parts of the procedure remained uneventful. However at the time of grafting of proximal ends of saphenous vein to the aorta we observed a partial collapse of the left lung and the ventilator bellows not inflating fully. This raised a suspicion of a leak in the breathing circuit but we did not find any circuit leak. An attempt was made to auscultate the chest but was not successful because the patient was draped with sterile towels. The anesthesia machine was crosschecked by a biomedical engineer but no technical error was detected. The inspiratory gas flow was increased to 3.0 liter/minute resulting in adequate expansion of the left lung, restoration of tidal volume and full inflation of ventilator bellows. Bulging of right side pleura was not observed on inspiration. During chest closure the heart rate (HR) increased to 130 beats/min with decrease in arterial blood pressure (ABP) to 80/60 mm Hg without any significant change in PIP, pulmonary artery pressure (PAP), 22/14 mm Hg and central venous pressure (CVP), 9 mm Hg. It was thought that the hemodynamic instability was due to the effect of chest closure and therefore was managed by administering fluid boluses and titrated increase in the dose of norepinephrine and epinephrine. At the end of the procedure, when all drapes were removed and dressing had been applied on the surgical wound while the patient was still on mechanical ventilation, there was an increase in PIP to 40 cm H2O. This was immediately followed by increase in HR to 150/min and a decrease in ABP to 60/40 mm Hg. We attempted to manage this episode as the previous one and transesophageal echocardiography (TEE) was called for. However there was no response to intravenous fluids and high doses of vasopressors. An increase in CVP to 14 mm Hg and PAP to 28/20 mm Hg with a decrease in cardiac index (CI) and cardiac output (CO) were observed. Auscultation of the chest revealed decreased breath sounds on the right side of the chest and crepitus was palpable over the neck and chest. This led to suspicion of subcutaneous emphysema and a diagnosis of tension pneumothorax was made. Immediately tube thoracostomy was performed on the right side and a gush Heart, Lung and Vessels. 2014, Vol. 6 205 A. Jain, et al. 206 of air bubbles were observed in the under water seal drainage system followed by normalization of heart rate, blood pressure and pulmonary artery pressure. TEE performed subsequently to rule out any signs of myocardial ischemia revealed normal LV function, adequately filled LV, no RWMA and no valvular regurgitation. The patient was then shifted to the postoperative intensive care unit and a chest radiograph obtained which revealed full lung expansion and mild subcutaneous emphysema. The trachea was extubated on the first postoperative day, chest tubes were removed on the third postoperative day without any evidence of pneumothorax on chest radiograph. Rest of the postoperative period was uneventful and the patient was discharged on the seventh postoperative day. DISCUSSION Tension pneumothorax is a life threatening condition and its occurrence intraoperatively should be promptly diagnosed and treated (3, 4). The most common causes are regional blocks (40% of reported cases), airway instrumentation (19%), barotrauma (16%), and placement of central venous lines (7%) (5). Patients with COPD are at increased risk (3). In our patient the cause of tension pneumothorax was thought to be rupture of an emphysematous bulla that might have been present on the right lung particularly since the chest radiograph showed emphysematous changes despite normal PFT. Moreover the patient was a chronic smoker and during surgery the lungs were observed to be hyperinflated. A communication between lung parenchyma and pleural space may act as a one way valve allowing air to enter inside the pleural cavity during inspiration but preventing the air from escaping naturally during expiration. This results in an expanding pneumothorax that forces the lungs to collapse, increases intrathoracic pressure that causes decrease in venous return to the heart, decrease in stroke volume, cardiac output, cardiac index, blood pressure and tachycardia eventually leading to hemodynamic compromise (6). McLoud et al. (7) reported a rise in PADP consistent with the development of pneumothorax in 3 patients (2 on mechanical ventilation). Yu and Lee (8) reported an increase only in PADP with pneumothorax and they considered it could be due to the transmission of the intrapleural pressure to the pulmonary vasculature. Connolly (9) reported the first and only description of a patient with tension pneumothorax in whom all hemodynamic and ABG parameters were measured. The authors described the onset of hypoxemia, acidosis, increased CVP, PAP and decrease of CO, consistent with the development of pneumothorax. Standard medical reference texts state that the immediate life-saving treatment for tension pneumothorax is needle decompression but there are case reports describing patients with tension pneumothorax managed successfully by chest tube drainage, without performing immediate needle decompression (10). Many experts would proceed directly to definitive treatment and bypass the step of needle decompression if the capability to perform tube thoracostomy is immediately available, and this is what we opted for. Classical signs of pneumothorax may be masked during general anesthesia. In mechanically ventilated patients, the physician may suspect tension pneumothorax when there is an increase in pleural pressures necessitating an increase in peak airway pressure in order to deliver the same TV. Decreased expiratory volumes secondary to air leakage into the pleural space and increased end-expiratory pressure, even after discontinuation of PEEP, are two other Heart, Lung and Vessels. 2014, Vol. 6 Tension pneumothorax during cardiac surgery signs of tension pneumothorax in these patients. Increased PAP and decreased CO or CI are other parameters suggestive of tension pneumothorax (6-9). Hemodynamic instability, hypoxia and/or increased oxygen requirements occur within minutes during positive pressure ventilation in comparison to hours during spontaneous respiration (11). In our case there were many signs indicating tension pneumothorax, such as a decrease in expiratory TV followed by increase in PADP, decrease in CO and CI leading to hemodynamic instability and lastly subcutaneous emphysema. However, since most of the signs also indicate hypovolaemia and contractile deficit that may occur frequently during OPCAB, this may lead to delay in diagnosis and treatment of the condition. We hypothesize that at the time of the first episode, start of a small pneumothorax resulted in reduction in minute volume without hemodynamic or airway pressure changes. The pneumothorax slowly progressed resulting in the second episode which was associated with hemodynamic changes but since the chest was still partially open the hemodynamics could be stabilized by fluid administration and inotropic support. However after chest closure the tension pneumothorax caused rapid, profound hemodynamic changes in the now closed chest cavity. We would like to highlight that intraoperative tension pneumothorax may definitely manifest after chest closure in cardiac surgical procedures. We conclude that the diagnosis of tension pneumothorax remains a challenge in mechanically ventilated patients under anesthesia. The presence of a cardiogenic shock-like picture, poor response to inotropes, increased inspiratory airway pressure, loss of tidal volume in a patient undergoing cardiac surgery may also be due to a tension pneumothorax. REFERENCES 1. Rojas R, Wasserberger J, Balasubramaniam S. Unsuspected tension pneumothorax as a hidden cause of unsuccessful resuscitation. Ann Emerg Med 1983; 12: 411-2. 2. Watts BL, Howell MA. Tension pneumothorax: a difficult diagnosis. Emerg Med J 2001; 18: 319-20. 3. Gold MI, Joseph SI. Bilateral tension pneumothorax following induction of anesthesia in two patients with chronic obstructive airway disease. Anesthesiology 1973; 38: 93-6. 4. Smith CE, Otworth JR, Kaluszyk P. Bilateral tension pneumothorax due to a defective anesthesia breathing circuit filter. J Clin Anesth 1991; 3: 229-34. 5. Ibrahim AE, Stanwood PL, Freund PR. Pneumothorax and systemic air embolism during positive-pressure ventilation. Anesthesiology 1999; 90: 1479-81. 6. Gustman P, Yerger L, Wanner A. Immediate cardiovascular effects of tension pneumothorax. Am Rev Respir Dis 1983; 127: 171-4. 7. McLoud TC, Barash PG, Ravin CE, Mandel SD. Elevation of pulmonary artery pressure as a sign of pulmonary barotrauma (Pneumothorax). Crit Care Med 1978; 6: 81-4. 8. Yu PYH, Lee LW. Pulmonary artery pressures with tension pneumotorax. Can J Anaesth 1990; 37: 584-6. 9. Connolly JP. Hemodynamic measurements during a tensión pneumotorax. Crit Care Med 1993; 21: 294-6. 10. Boon D, Llewellyn T, Rushton P. A strange case of tension pneumothorax. Emerg Med J 2002; 19: 470-471. 11. Derek J Roberts, Simon Leigh-Smith, Peter D Faris, Chad G Ball, Helen Lee Robertson, Christopher Blackmore, et al. Clinical manifestations of tension pneumothorax: protocol for a systematic review and meta-analysis. Systematic Reviews 2014; 3: 3. Cite this article as: Jain A, Arora D, Juneja R, Mehta Y, Trehan N. Life threatening tension pneumothorax during cardiac surgery. A case report. Heart, Lung and Vessels. 2014; 6(3): 204-207. Source of Support: Nil. Disclosures: None declared. Heart, Lung and Vessels. 2014, Vol. 6 207 IMAGES IN MEDICINE Heart, Lung and Vessels. 2014; 6(3): 208-209 208 Coronary to extra-cardiac anastomosis A. Mohsen, J. Loughran, S. Ikram Division of Cardiology, University of Louisville, KY, USA Keywords: collaterals, coronary to extra-cardiac anastomosis, coarctation of the aorta. We are reporting a rare case of a patient with the right coronary artery giving a large collateral vessel to an intercostal artery in a patient with repaired coarctation of aorta. A 64 year-old man with coarctation of the aorta, surgically repaired at 18 years of age, presented with dyspnea. A trans-esophageal echocardiogram revealed a severely stenotic bicuspid aortic valve. A Corresponding author: Sohail Ikram, M.D. University of Louisville Department of Cardiology 550 South Jackson Street ACB, 3rd floor Louisville, KY 40202 e-mail: s0ikra02@louisville.edu A coronary angiogram was performed prior to valve surgery. A left anterior oblique caudal cineangiographic view of the coronary anatomy revealed a very long collateral vessel arising from the conus branch of the right coronary artery that appeared to insert into a hypertrophied blood vessel terminating in the left thorax. The distal portion of this vessel exhibited minimal motion with ventricular contraction, and appeared fixed to the chest wall, suggesting this was a prominent intercostal artery (Figures 1 A, B). B Figures 1 A, B - Left anterior oblique caudal cineangiographic view of the coronary anatomy revealing a very long collateral vessel arising from the conus branch of the right coronary artery that appeared to insert into a hypertrophied blood vessel terminating in the left thorax. The distal portion of this vessel exhibited minimal motion with ventricular contraction, and appeared fixed to the chest wall suggesting this was a prominent intercostal artery. Heart, Lung and Vessels. 2014, Vol. 6 Coronary to extra-cardiac anastomosis pensatory mechanism to bypass the stenosis in patients with aortic coarctation (1). Collaterals from the thyrocervical trunk, thoracic arteries arising from the axillary artery and the internal mammary arteries are most commonly observed (2). Coronary collateral circulation within the heart is a well-known phenomenon (3). Extracardiac-to-coronary anastomosis is gaining more attention (4). The most common types of extracardiac-tocoronary anastomoses are from the internal mammary artery and the bronchial arteries. Both typically occur in the presence of a chronic occlusion of a coronary artery. To our knowledge this is the first reporting of coronary-to-extracardiac anastomoses. Figure 2 - Left anterior oblique cranial view of the aortic arch demonstrating dilated vessels arising from the arch proximal to the surgically corrected stenosis. These findings are consistent with clinical history of coarctation of aorta. The left anterior oblique cranial view of the aortic arch demonstrated dilated vessels arising from the arch proximal to the surgically corrected stenosis (Figure 2). This collateralization is an imperative com- REFERENCES 1. Gross RE. Coarctation of the aorta. Circulation. 1953. 5:757-68. 2. Keane JF, Flyer DC. Coarctation of the aorta. In: Nadas’ Pediatric Cardiology, 2nd Ed., Keane JF, Lock JE, and Fyler DC. (Eds), Saunders Elsevier, Philadelphia 2006; 627. 3. Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C. Assessment of the human coronary collateral circulation. Circulation. 2010; 122: 1210-20. 4. Unger EF, Sheffield CD, Epstein SE. Creation of anastomoses between an extracardiac artery and the coronary circulation. Proof that myocardial angiogenesis occurs and can provide nutritional blood flow to the myocardium. Circulation. 1990. 82: 1449-66. Cite this article as: Mohsen A, Loughran J, Ikram S.Coronary to extra-cardiac anastomosis. Heart, Lung and Vessels. 2014; 6(3): 208-209. Source of Support: Nil. Disclosures: None declared. Heart, Lung and Vessels. 2014, Vol. 6 209 IMAGES IN MEDICINE Heart, Lung and Vessels. 2014; 6(3): 210-212 210 Internal thoracic vein: friend or foe? A. Roubelakis, D. Karangelis, S.K. Ohri Department of Cardiothoracic Surgery, Southampton University Hospitals NHS FoundationTrust, Southampton, UK Keywords: graft elongation, coronary artery bypass, revascularization. The internal thoracic vein is a conduit that has not been thoroughly investigated in literature as long term patency and outcomes are unknown. We present a case where the right internal thoracic vein (RITV) was used to extend a short right internal thoracic artery (RITA). The elongated composite conduit was then anastomosed to the right coronary artery (RCA). A 61-year-old male patient was referred electively for a coronary artery bypass grafting (CABG) operation. The patient had undergone extensive stenting to the left and right coronary artery systems. Unfortunately the stents to the right coronary artery had restenosed causing significant symptoms necessitating revascularization (Figure 1). From the conduit point of view, the patient had his long saphenous veins fully stripped bilaterally. His radial arteries were assessed with Allen’s test and use of saturation monitor: following the occlusion of the radial the saturations failed to rise, therefore they were deemed unusable. RITA was therefore elected to be the conduit of choice. Corresponding author: Dimos Karangelis MD, PhD Wessex Cardiac Centre Southampton University Hospitals FoundationT NHS Trust Tremona Road, Hampshire, UK e-mail: dimoskaragel@yahoo.gr Figure 1 - Preoperative angiogram showing an instent occlusion of the mid-distal RCA. RCA = right coronary artery. The operation was performed in a standard on-pump fashion. RITA was harvested initially as a pedicled graft. The target vessel was measuring approximately 1.5 mm in diameter and was opened distally due to the presence of the previous stents and the anatomy of the lesions. Unfortunately RITA intima was found to be of suboptimal quality and calibre distally and had to be shortened. This resulted in RITA length being insufficient to reach the target vessel, even with skeletonisation. RITA was then extended with a 2-3 cm segment of RITV which appeared to be of good quality and calibre. The reversed RITV and RITA were anastomosed in an end-to-end fashion using continuous 8-0 polypropylene suture (Figure 2). The composite graft was then anastomosed Heart, Lung and Vessels. 2014, Vol. 6 Internal thoracic vein: friend or foe? 211 Figure 2 - The end to end anastomosis (circled) between the RITA and RITV. There is no mismatch in the calibre of the two vessels. RITA=right internal thoracic artery; RITV = right internal thoracic vein. Figure 3 - The composite conduit as viewed from the patient’s head after the completion of the distal anastomosis. distally to the RCA (Figure 3) and proximally to the ascending aorta. There was excellent flow down this graft. The operation was completed uneventfully and the patient was discharged home on the 5th postoperative day. He remains symptomfree at a 6 month follow up. The ITV conduit studies in literature are very limited as they have not being used routinely, therefore long term results are unknown. The use of any other venous conduit (like short saphenous or cephalic veins) was not preferred due to patient’s age and would also lead to significant size mismatch between the RITA and the vein, if used as extentions. Left internal thoracic artery (LITA) needed to be preserved for possible future revascularization on the left coronary system. The use of gastroepiploic artery is not performed routinely in our unit and therefore experience is very limited. There is only one study in literature where ITVs were used as CABG grafts to the left anterior descending artery in a minipig model. The authors measured significant intimal hyperplasia in these grafts after 4 weeks (1). Similar studies to humans however have not been performed. A report from 1990 presented a 57 year old Heart, Lung and Vessels. 2014, Vol. 6 A. Roubelakis, et al. 212 patient who underwent CABG operation with the use of left and right ITAs and internal mammary vein (IMV). to an obtuse marginal. Angiography after 10 days revealed excellent patency of the IMV graft (2). It is difficult to justify the use of internal thoracic veins as conduits for CABG, as in most cases there is ample selection of other well established conduits. The calibre of these veins is similar to internal mammary arteries (IMAs) and could potentially be used as extensions. ITA elongation has been described before with the utilization of various grafts (3,4). To summarise, our method could potentially be useful when the internal mammary artery is not long enough to reach the target vessel when no other option is possible. The major limitation of this report is that a postoperative angiogram to assess the patency of the graft could not be obtained. There is no need of ethical committee approval for this case report. Written informed consent was obtained from the patient. REFERENCES 1. Popov AF, Dorge H, Hinz J, Schmitto JD, Stojanovic T, Seipelt R, et al. Accelerated intimal hyperplasia in aortocoronary internal mammary vein grafts in minipigs. J Cardiothorac Surg. 2008; 3: 20. 2. Stephan Y, Jebara VA, Fabiani JN, Carpentier A. The internal mammary vein: a new conduit for coronary artery bypass. J. Thorac. Cardiovasc. Surg. 1990; 99: 178. 3. Calafiore AM, Teodori G, Di Giammarco G, Vitolla G, Contini M, Maddestra N, et al. Left internal mammary elongation with inferior epigastric artery in minimally invasive coronary surgery. Eur J Cardiothorac Surg. 1997; 12: 393-6; discussion 397-8. 4. Bernet FH, Hirschmann MT, Reineke D, Grapow M, Zerkowski HR. Clinical outcome after composite grafting of calcified left anterior descending arteries. J Cardiovasc Surg (Torino) 2006; 47: 569-74. Cite this article as: Roubelakis A, Karangelis D, Ohri SK. Internal thoracic vein: friend or foe? Heart, Lung and Vessels. 2014; 6(3): 210-212. Source of Support: Nil. Disclosures: None declared. Acknowledgment: We thank Anne Gale for editorial assistance. Heart, Lung and Vessels. 2014, Vol. 6 FUTURE EVENTS Calendar for future meetings Intensive Care, Surgery and Cardiovascular Anesthesia 2014 October 4-7. Congress Australian Society of Anesthesiologists. Gold Coast, Australia. Info: www.asa2014.com.au October 11-15. 28th Annual Meeting European Association of Cardiothoraic Surgery. Milan, Italy. Info: www.eacts.org October 12. Roland Hetzer International Cardiothoracic and Vascular Surgery Society (RHICS) 8th Expert Forum during the 28th annual EACTS Meeting, Milan Italy. Info: rhics@dhzb.de October 11-15. ASA Annual Meeting. New Orleans. LA Info: www.asahq.org October 26-30. American College of Surgeons Clinical Congress. San Francisco, CA. Info: kmatousek@facs.org November 5-8. 61st Annual Meeting STSA. Tucson, AZ. Info: mdrumm@stsa.org November 6-8. 91st Annual Scientific Meeting of the Korean Society of Anesthesiologists. Seoul, Korea. Info: www.ksa-conference.org November 6-8. Annual Scientific Meeting Australian and New Zealand College of Perfusionists. Auckland, New Zealand. Info: Taryn@adhb.govt.nz November 14. ESA Focus Meeting on Peri-Operative Medicine: The pediatric patient. Athens, Greece. www.info@esahq.org November 29. Roland Hetzer International Cardiothoracic and Vascular Surgery Society (RHICS) 9th Expert Forum. In cooperation with the 9th Asian Cardiothoracic Surgery Specialty Update Course of Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong. Info: rhics@dhzb.de December 11-13. 6th International Congress: Aortic Surgery and Anesthesia “How to Do It”. Italy. Info: www.aorticsurgery.it December 12-16. Postgraduate Assembly, New York State Society of Anesthesiologists. New York, NY. Info: www.nyssa-pga.org 2015 January 17-21. 44th Congress Critical Care Medicine. Phoenix, Arizona. Info: www.sccm.org January 18-24. 33rd Annual Symposium: Clinical Update in Anesthesiology, Surgery and Perioperative Medicine. Four free workshops. St. Kitts, West Indies. Info: george.silvay@mountsinai.org February 11-15. 35th Annual Cardiothoracic Surgery Symposium CREF. San Diego, CA. Info: www.crefmeeting.com March 1-6. 30th International Symposium Interventional Cardiology. Snowmass, CO. Info: rlaw@promedicacme.com March 17-20. 35th International Symposium on Intensive Care and Emergency Medicine. Brussels, Belgium. Info: www.intensive.org March 21-24. 35th International Symposium on Intensive Care and Emergency Medicine. Brussels, Belgium. Info: www.intensive.org March 21-24. International Anesthesia Research Society (IARS), Honolulu, Hawaii. Info: www.iars.org/2015meeting April 15-18. 8th International Symposium: Diabetes, Hypertension, Metabolic Syndrome. Berlin, Germany. Info: www.comtecmed.com/DIP April 25-29. 95th Annual Meeting AATS. Seattle, Washington. Info: www.aats.org April 30-May 2. 62nd Annual Meeting Association of University Anesthesiologists. Nashville, TN. Info: www.AUAhq.org May 28-June 2. ESA Annual Meeting, Berlin, Germany. Info: www.info@esahq.org June 4-6.3rd International Symposium: Perioperative Care for Seniors. Prague, Czech Republic. Info: www.anesthesiaforsenior2015.cz June 11-12. EACTA Annual Meeting. Gothenburg, Sweden. Info: anne.westerlind@medfak.se August 29-September 1. World Federation of Societies of Critical Care Medicine, Seoul, Korea. Info: www.wfsiccm2015.com September 17-20. Canadian Association of Thoracic Surgeons. Montreal, Canada. Info: cats@canadianthoracicsurgeons.ca October 24-28. ASA Annual Meeting. San Diego, CA. Info: www.asahq.org November 9-10. Surgery of the Aorta. Bologna, Italy. Info: www.noemacongressi.it December 11-15. Sixty-Ninth Postgraduate Assembly. New York Stat of Anesthesiologists. New York, NY. Info: www.nyssa-pga.org 2016 January 16-23. 34th Annual Symposium: Clinical Update in Anesthesiology, Surgery and Perioperative Medicine. San Juan. Puerto Rico. Info: George.silvay@mountsinai.org March 22-25. 36th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, Info: www.intensive.org August 28-September 2. 16th World Congress of Anesthesiologists Hong Kong. Info: www.WCA2016.com 2020 September. 17th World Congress of Anesthesiologists. Prague, Czech Republic. Info: www.csarim2020.cz “Heart, Lung and Vessels” welcomes announcements of interest to physicians, researchers and others concerned with cardiothoracic and vascular surgery, anesthesiology, medicine, pharmacology and related areas. All copies are reviewed and edited for style, clarity and length. Information is due at least 90 days before the date of publication, and should be addressed to: George Silvay, M.D., Ph.D., Editor, Professor of Anesthesiology, Department of Anesthesiology, Mount Sinai Medical Center, 1 Gustave L. Levy Place, Box 1010, New York, NY 10029-6574. Email: george.silvay@mountsinai.org Heart, Lung and Vessels. 2014, Vol. 6 213 How to prepare a manuscript for submission to Heart, Lung and Vessels English is the only language allowed for manuscripts submitted to “Heart, Lung and Vessels”. You can choose between American and British English, but please be consistent. “Heart, Lung and Vessels” is an International Journal free of charge and with no registration. Please send your manuscript to Lara Sussani editorialoffice@heartlungandvessels.org. The Editorial Office will send to the corresponding author by e-mail all communications relating to the status of a submission, including the final decision and the scheduled date of publication. Manuscripts for the following sections may be submitted to “Heart, Lung and Vessels”: Original Articles; Teaching Articles; Brief Reports; Reviews; Meta-Analyses; Expert Opinions; Editorials; Case Reports, Case Series; Letters to the Editor; Images in Clinical Medicine. The peer-review process is applied to all submissions, including appraisal by at least two peer-reviewers. Manuscripts should be prepared in a standard word-processing program using Arial or Times New Roman font size 12 and double spacing. Pages must be numbered. Word limits are not imposed on any manuscript types, but all papers should be as concise as possible. COVER LETTER. Your manuscript should be accompanied by a cover letter addressed to the Editor in Chief and signed by the corresponding author. The letter must include at the end a list of all authors. The cover letter must state that all authors agree with and are responsible for the data presented. The letter should also acknowledge or deny any potential conflicts of interest. MANUSCRIPT. Our preferred file type for manuscript submissions is a single Microsoft Word document containing all elements of the article – text, references, tables and figure legends - except figures. Figures should be sent as separate files (see below). Print the title of the paper on the first page (TITLE PAGE); the title should be concise. Also provide a concise shortened title for the running head. Give the first name, surname, the department and institutional affiliation of each author. Specify the name, address, and e-mail address of the author to whom correspondence should be addressed. ABSTRACT: Provide an abstract of not more than 250 words and without abbreviations. Abstracts of original articles and meta-analyses should consist of four paragraphs labeled Intro- duction, Methods, Results and Conclusions. They should briefly describe, respectively, the problem being addressed in the study, how the study was performed, the salient results and what the authors conclude from the results. Abstracts of other article types should consist of a single paragraph of not more than 250 words and without titles or abbreviations. Original manuscript text must be divided into five sections: Introduction, Methods, Results, Discussion and Conclusion. Review articles may have different sections. REFERENCES. Please refrain from using automatic reference list software because its features are often lost during the publication process. Simply insert the reference number in round parentheses in the text (before any punctuation mark) and type the reference list. References must be numbered with Arabic numerals and cited in the text in numerical order (the first reference that appears in the text is number 1, the second reference that appears in the text is number 2 and so on). The reference list at the end of the article must also be in numerical order. Please cite references, tables and figures in the text in round brackets. The list, headed “References”, should begin on a new page of the main text document and be double-spaced. Give all authors when there are six or fewer; when there are more than six, list the first six, followed by “et al.”. Abbreviations for titles of medical periodicals must conform to those used in Index Medicus (www.nlm.nih.gov/tsd/serials/lji. html). References to abstracts, supplements and letters to editors must be identified as such. Inclusive page numbers of references are required. Original article: no more than 30 references, Review article: no more than 50, Editorial and Case Report: no more than 5. DETAILS. “Heart, Lung and Vessels” will consider for publication suitable articles on all topics related to cardiothoracic and vascular surgery, intensive care and anesthesia together with cardiology, emergency and methodological papers. The aim of “Heart, Lung and Vessels” is to contribute to the spread of knowledge in the field of intensive care, emergency treatment and major surgical operations. Manuscripts are examined by members of the editorial staff and then sent to outside reviewers. We encourage authors to suggest the names of possible reviewers, but we reserve the right of final selection. Communications about manuscripts will be sent after the review when the editorial decisionmaking process is complete. 215 216 All articles represent the opinion of the authors and do not necessarily reflect the opinion of the Editor, Editorial Board or Publisher. The Editors and Publisher deny any responsibility or liability for statements and opinions expressed by the authors. Neither the Editor nor the Publisher guarantee, warrant or endorse any product or service advertised in this publication, nor do they guarantee any claim made by the manufacturer of such product or service. Manuscripts containing original material are accepted for consideration if neither the article nor any part of its essential substance, tables, or figures have been or will be published or submitted elsewhere before appearing in “Heart, Lung and Vessels” This restriction does not apply to abstracts or press reports published in connection with scientific meetings. Authors of all types of articles should follow the instructions given here. These guidelines are in accordance with the “Uniform Requirements for Manuscripts Submitted to Biomedical Journals” published by the International Committee of Medical Journal Editors at www.icmje.org Papers reporting human experimentation will be reviewed in accordance with the precepts established by the Helsinki Declaration (www.wma.net/ en/30publications/10policies/b3/17c.pdf). Copies of this declaration may also be obtained by writing to the American Medical Association, 515 N State St, Chicago, IL 60610, USA. As stated in the Uniform Requirements, credit for authorship requires substantial contributions to (a) the conception and design of the protocol or analysis and interpretation of the data and (b) the drafting of the article or critical revision for important intellectual content. Each author must sign a statement attesting that he or she fulfills the authorship criteria of the Uniform Requirements. Any change in authorship after submission must be approved in writing by all authors. In appropriate places in the manuscript, please provide, if applicable, a statement that the research protocol was approved by the relevant institutional review boards or ethics committees and that all human participants gave written informed consent. When reporting experiments on human subjects, authors should indicate whether the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. If doubt exists whether the research was conducted in accordance with the Helsinki Declaration, the authors must explain the rationale for their approach, and demonstrate that the institutional review body explicitly approved the doubtful aspects of the study. When reporting experiments on animals, authors should indicate whether the institutional and national guide for the care and use of laboratory animals was followed. If photographs of patients are used, either they should not be identifiable or the photographs should be accompanied by written permission to use them. Patients have a right to privacy that should not be infringed without informed consent. Identifying information, including patients’ names, initials, or hospital numbers, should not be published in written descriptions, photographs, and pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian) gives written informed consent for publication. Authors should identify individuals who provide writing assistance and disclose the funding source for this assistance. Figures. Figures should be sent as separate files. It is permissible to send low-resolution figures for peer review. Please note that if the manuscript is accepted for publication we will ask for high-resolution files of all figures: - figures containing only lines and/or text (such as graphs or flowcharts) must have a resolution of 900 dpi AND a width between 100 and 200 mm; - figures containing both lines/text and images (such as mixed figures containing graphs and images) must have a resolution of 600 dpi AND a width between 100 and 200 mm; - figures not containing lines or text (i.e. color or black and white images) must have a resolution of 300 dpi AND a width between 100 and 200 mm. Formats accepted are JPEG (.jpg), TIFF (.tif), PNG (.png). Images with a resolution of 72 dpi or smaller than 100 mm will not be accepted. Legends for all figures should be included in the file with the text (on a new page after the reference list) and should not appear on the figures themselves. Tables. Tables should be prepared in Microsoft Word; they should be formatted preferably with the tabulation command, otherwise as Microsoft Word tables. Do not submit tables in PDF format or any graphic format. Abbreviations. Except for units of measurement, abbreviations are strongly discouraged. The first time an abbreviation appears, it should be preceded by the words for which it stands (except for units of measurement). No abbreviations are allowed in the abstract. Drug names. Generic names should be used. When proprietary brands are used in research, include the brand name and the name of the manufacturer in parentheses after the first mention of the generic name in the Methods section. Instructions for submitting a revised manuscript. We require two versions of the revised manuscript, one with “tracked” or highlighted changes and one without. Please double-space. Include your response to the reviewers as a separate file. If a submitted article is accepted for publication, editorial revisions may be made to aid clarity and understanding without altering the meaning. CONFLICT OF INTEREST. Public trust in the peer review process and the credibility of published articles depend in part on how well conflict of interest is handled during writing, peer review, and editorial decision-making. Conflict of interest exists when an author (or the author’s institution), reviewer, or editor has financial or personal relationships that inappropriately influence (bias) his or her actions (such relationships are also known as dual commitments, competing interests, or competing loyalties). These relationships vary from those with negligible potential to those with great potential to influence judgment, and not all relationships represent true conflict of interest. The potential for conflict of interest can exist whether or not an individual believes that the relationship affects his or her scientific judgment. Financial relationships (such as employment, consultancies, stock ownership, honoraria, paid expert testimony) are the most easily identifiable conflicts of interest and the most likely to undermine the credibility of the journal, the authors, and of science itself. However, conflicts can occur for other reasons, such as personal relationships, academic competition, and intellectual passion. “Heart, Lung and Vessels” expects that all authors acknowledge financial associations with a company (or its competitor) that makes a product discussed in the article. Information published in medical journals helps shape diagnostic and therapeutic decisions. For a journal to be of value, it must publish authoritative, up-to-date information that is free of commercial influence. Because relationships between authors and biomedical companies are growing, we want to ensure that the articles we publish are not influenced by financial interests. Authors should disclose any financial arrangement they may have had in the last 3 years or will have in the foreseeable future with a company whose product is pertinent to the submitted manuscript or with a company making a competing product. Such information will be held in confidence while the paper is under review and will not influence the editorial decision but, if the article is accepted for publication, a disclosure statement will appear with the article. Here are some examples: Dr. “A” reports having served as a consultant to “A1.” Dr. “B” reports having been paid lecture fees by “B1”, “B2” and “B3.” Drs. “C, D, E” report having received grant support from “C1.” Neither Dr. “F” nor Dr. “G” has any financial interest in the patent. Dr. “H” and Dr. “I” are consultants to “H1.” Dr. “L” reports having received consulting fees from “I1.” Dr. “M” reports having been a member of speakers’ bureaus sponsored by “M1.” COPYRIGHT. “Heart, Lung and Vessels” is the owner of all copyright to any published work. “Heart, Lung and Vessels” and its licensees have the right to use, reproduce, transmit, derive works from, publish and distribute the contribution in the “Heart, Lung and Vessels” or otherwise in any form or medium. Authors may not use or authorize the use of the contribution without the written consent of “Heart, Lung and Vessels”. EDITING SERVICES “Heart, Lung and Vessels” offers high quality editing service of English language revision and statistical and methodological support, applying convenient low rates to authors who wish to publish in peer-reviewed journals. While no guarantee for article acceptance can be made, improved English structure, appropriate scientific methodology and statistical revision can greatly enhance the readability and message of a paper, increasing its possibility to be published in major international peer reviewed journals. For information and tariffs please contact Lara Sussani editorialoffice@heartlungandvessels.org Please direct any questions to editorialoffice@ heartlungandvessels.org or visit http://www.heartlungandvessels.org/ “Heart, Lung and Vessels” editorial offices are located in the Department of Anesthesia and Intensive Care at 60 Via Olgettina, Milan, Italy 20132, tel. (+39) 02.26436158, fax (+39) 02.26436152, Lara Sussani email: editorialoffice@ heartlungandvessels.org 217