Journal Club Slides
advertisement
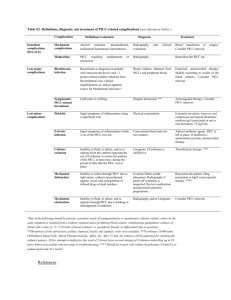
JAMA Pediatrics Journal Club Slides: Postdischarge Treatment of Acute Osteomyelitis Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. Published online December 15, 2014. doi:10.1001/jamapediatrics.2014.2822. Copyright restrictions may apply Introduction • Background – Postdischarge treatment of acute osteomyelitis in children requires weeks of antibiotic therapy. – Therapy can be administered orally or intravenously via a peripherally inserted central catheter (PICC). – PICCs carry a risk for serious complications. – There is limited evidence about the effectiveness of oral therapy. • Study Objective – To compare the effectiveness and adverse outcomes of postdischarge antibiotic therapy administered via the PICC or the oral route. Copyright restrictions may apply Methods • Study Design – Retrospective cohort study. • Setting – 36 US children’s hospitals. • Patients – Children hospitalized from January 1, 2009, through December 31, 2012. • Intervention – Postdischarge administration of antibiotics via the PICC or the oral route. Copyright restrictions may apply Methods • Primary Outcome – Treatment failure, defined as revisit to the emergency department (ED) or a rehospitalization for the following: • Change in the antibiotic prescribed or its dosage. • Prolongation of antibiotic therapy. • Conversion from the oral route to the PICC route. • Bone abscess drainage. • Debridement of necrotic bone. • Bone biopsy. • Drainage of an abscess of the skin or muscle. • Arthrocentesis. • Diagnosis of a pathologic fracture. Copyright restrictions may apply Methods • Secondary Outcomes – Return to the ED or a rehospitalization for adverse drug reaction (vomiting and/or diarrhea, dehydration, Clostridium difficile infection, allergic reaction, urticaria, anaphylaxis, drug-induced neutropenia, acute kidney injury, Stevens-Johnson syndrome, erythema multiforme, or other). – Return to the ED or a rehospitalization for PICC complication (fever evaluation, infection at the site of the PICC insertion, bloodstream infection, sepsis, thrombosis, or breakage, repair, adjustment, manipulation, or removal of the PICC line with or without insertion of a new line). – Composite of treatment failure, adverse drug reaction, and PICC line complication. Copyright restrictions may apply Methods • Propensity Score–Based Full Matching – Method for dealing with confounding by indication. – Propensity score calculated from logistic regression that includes age, race, insurance, length of stay, infection location, surgical procedures (arthrocentesis, osteotomy, soft-tissue incision and drainage, and arthrotomy), and causative pathogen. – Full matching links each child who received antibiotics via the PICC route to the most similar child who received antibiotics via the oral route or vice versa (based on propensity score). – Matching within hospitals controls for hospital-level confounders, analogous to cluster randomized trial. – Matching across hospitals controls better for patient-level confounders. • Effect Modification – Age, culture-verified presence of methicillin-resistant Staphylococcus aureus (MRSA), ie, is the effectiveness different in younger children, or children with MRSA? Copyright restrictions may apply Results Flowchart of the Study Cohort Copyright restrictions may apply Results Scatterplot of Hospital Volume of Osteomyelitis, Proportion of Children With Postdischarge Antibiotic Therapy Via the PICC Route, and Their Crossclassification Across Hospitals • Large and evenly distributed variation in use of PICCs. • No correlation between hospital volume of osteomyelitis and use of PICCs. Copyright restrictions may apply Results Clinical and Demographic Characteristics of the Study Population • Small differences between the oral and PICC routes in a few key variables. Copyright restrictions may apply Results Clinical and Demographic Characteristics of Treatment Groups After Within- and AcrossHospital Matching • Matching reduces differences. Copyright restrictions may apply Results Adverse Outcomesa Copyright restrictions may apply Results • Effect Modification – No clinically relevant difference in rates of treatment failure for children 5 years and younger. • Across-hospital risk difference = −1.3% (95% CI, −4.7% to 2.1%). • Within-hospital risk difference = −2.8% (95% CI, −7.4% to 1.7%). – Risk for treatment failure increased in children older than 5 years who had received antibiotics via the PICC route. • Across-hospital risk difference = 3.8% (95% CI, 1.3%-6.3%). • Within-hospital risk difference = 4.6% (95% CI, 2.2%-7%). – Isolation of MRSA as the causative organism did not modify the effect of the treatment route on the outcome of treatment failure. Copyright restrictions may apply Comment • Children discharged to complete antibiotic course via oral route did NOT have a higher rate of treatment failure. • Children with MRSA infections did not have more treatment failures. • High rate of serious complications of PICC line (15% requiring ED revisit or rehospitalization): bloodstream infections, thromboembolism, line displacement or breakage. Copyright restrictions may apply Comment • Likely to be strongest evidence available to answer question. • Randomized clinical trial not feasible. • Confirms results of prior study that used only administrative data. • Results consistent, even with increase in MRSA prevalence (study period 2009-2012). • Stop using PICC lines to treat acute osteomyelitis in otherwise healthy children who can tolerate oral route. • Oral route is equally effective, has fewer complications, is less expensive, and is more convenient. Copyright restrictions may apply Contact Information • If you have questions, please contact the corresponding author: – Ron Keren, MD, MPH, Division of General Pediatrics, Center for Pediatric Clinical Effectiveness, Children’s Hospital of Philadelphia, Abramson Research Building, Room 1347, Philadelphia, PA 19104 (keren@email.chop.edu). Funding/Support • This study was supported by the Patient Centered Outcomes Research Institute. Conflict of Interest Disclosures • None reported. Copyright restrictions may apply