New Paradigms in the
Science and Medicine of Heart Disease
Deciphering the Maze of Risk-Specific
Interventions for Stroke Prevention in
Atrial Fibrillation
What Do Late-Breaking Trials Teach Us About
Optimal Alignment of New and Established Therapies for
Risk-Stratified Subgroups with AF?
Program Chairman
Samuel Z. Goldhaber, MD
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
Program Faculty
Program Chairman
Samuel Z. Goldhaber, MD
Jeffrey I. Weitz, MD, FRCP, FACP
Elaine M. Hylek, MD, MPH
Associate Professor of Medicine
Department of Medicine
Boston University Medical Center
Boston, MA
Jonathan L. Halperin, MD
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
David A. Garcia, MD
Associate Professor, Division of General
Internal Medicine
University of New Mexico
Co-Director, University of New Mexico
Anticoagulation Management Service
President, Anticoagulation Forum
Professor of Medicine and Biochemistry
McMaster University
Director, Henderson Research Center
Canada Research Chair in Thrombosis
Heart and Stroke Foundation
J.F. Mustard Chair in Cardiovascular Research
Hamilton, Ontario, Canada
Professor of Medicine (Cardiology)
Mount Sinai School of Medicine
Director, Clinical Cardiology Services
The Zena and Michael A. Weiner
Cardiovascular Institute
The Marie-Josée and Henry R. Kravis Center
for Cardiovascular Health
New York, NY
New Frontiers in Atrial Fibrillation
ATRIAL FIBRILLATION
Current Dilemmas in Stroke Prevention
Challenges in Risk-Specific Intervention for AF—
”Taking the Pulse” for Year 2010 and Beyond
Samuel Z. Goldhaber, MD
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
Adverse Events: Atrial Fibrillation
1. Death
2. Stroke
3. Hospitalizations
4. Quality of life and exercise capacity
5. Left ventricular dysfunction
European Heart Journal 2010; 31: 2369-2429
Atrial Fibrillation: Twice as Common
as Previously Suspected
► Incidence increased 13% over past
20 years
► In USA, 12-16 million will be
affected by 2050
► Increasing obesity and increasing
age are risk factors that help explain
rise in incidence
Miyasaka Y. Circulation 2006; 114: 119-125
AF Prevalence: Age and Gender
Prevalence, percent
Prevalence of AF
increases with age;
men > women
Age, years
JAMA 2001; 285: 2370
The Percentage of Strokes
Attributable to AF Increases with Age
Framingham Study
30
20
AF prevalence
%
Strokes attributable
to AF
10
0
50–59
60–69
70–79
80–89
Age Range (years)
Wolf et al. Stroke 1991; 22: 983-988
Mortality Rates in AF
► Double the overall age and gender
matched population
► No reduction in past two decades
► Mortality 9-fold higher during 1st 4
months after diagnosis
Miyasaka Y, et al. JACC 2007; 49: 986-992
Risk Factors for Stroke
Risk Factor
Relative Risk
Old Stroke/TIA
2.5
Hypertension
1.6
CHF
1.4
Increased age
1.4/10 years
DM
1.7
CAD
1.5
Arch Intern Med 1994; 154: 1449-1457
Atrial Fibrillation:
A Risk Factor for Vascular Events
•
•
•
•
•
RISK FACTORS: THROMBOSIS
Hypertension
Hyperlipidemia
Age
Diabetes Mellitus
Smoking
Atherosclerosis/Atherothrombosis
Atherosclerosis/Atherothrombosis
MI
MI
AF
Stroke, MI, Vascular Death
Wolf PA et al. Arch Intern Med 1987; 147: 1561-1564
Leckey R et al. Can J Cardiol 2000; 16: 481-485
CHF
CHF
Therapeutic Range for Warfarin
INR Values at Stroke or ICH
Odds Ratio
15.0
Stroke
Intracranial
Hemorrhage
10.0
5.0
1.0
0
1.0
2.0
3.0
4.0
Fuster et al. J Am Coll Cardiol. 2001;38:1231-1266.
INR
5.0
6.0
7.0
8.0
Problems with Warfarin
1. Delayed onset/offset
2. Unpredictable dose response
3. Narrow therapeutic index
4. Drug-drug, drug-food interactions
5. Problematic monitoring
6. High bleeding rate
7. Slow reversibility
The Current Landscape
Novel Oral Anticoagulants
Novel Oral Anticoagulants
1. Dabigatran: An oral DTI—twice daily (renal
clearance)
2. Rivaroxaban: Direct factor Xa inhibitor
(renal clearance)—once or twice daily
3. Apixaban: Direct factor Xa inhibitor
(hepatic clearance)—twice daily
4. Edoxaban: Direct factor Xa inhibitor
(hepatic clearance)—once daily
Circulation 2010; 121: 1523-1532
RE-LY: A Non-inferiority Trial
Atrial fibrillation
≥1 Risk Factor
Absence of contra-indications
951 centers in 44 countries
Blinded Event Adjudication
R
Open
Warfarin
adjusted
(INR 2.0-3.0)
N=6000
NEJM 2009; 361:1139-51
Blinded
Dabigatran
Etexilate
110 mg BID
N=6000
Dabigatran
Etexilate
150 mg BID
N=6000
Stroke or Systemic Embolism
Non-inferiority
p-value
Superiority
p-value
Dabigatran 110 vs. Warfarin
<0.001
0.34
Dabigatran 150 vs. Warfarin
<0.001
<0.001
Margin = 1.46
0.50
0.75
HR
NEJM 2009; 361:1139-51
Dabigatran better
1.00
1.25
(95% CI)
1.50
Warfarin better
Intracranial Bleeding
RR 0.31 (95% CI: 0.20–0.47)
p<0.001 (sup)
RR 0.40 (95% CI: 0.27–0.60)
p<0.001 (sup)
Number of events
0.74 %
RRR
60%
RRR
69%
0.30 %
0.23 %
Connolly et al., NEJM, 2009; 361: 1139-1151
Mortality Rate:
Dabigatran Compared To Warfarin
Dabigatran: 15% reduction in death from
vascular causes (p=0.04)
12% reduction in death from any
cause (p=0.05)
NEJM 2009; 361: 1139-1151
FDA Approves Dabigatran for SPAF
Dabigatran Reduces Stroke Rate Compared to Warfarin
October 19, 2010
►
Dose of 75 mg BID for CrCl 15-30 ml/ min
►
Dose of 150 mg BID for CrCl > 30 ml/ min
Deciphering the Maze of RiskSpecific Interventions for SPAF
► ROCKET-AF results just released at noon
today—Dr. Weitz will present top-line in his
presentation and Dr. Hylek will address
implications in her case studies
► Will we be able to make “cross-trial”
comparisons between RE-LY and ROCKET-AF?
Analysis just beginning . . .
Deciphering the Maze of RiskSpecific Interventions for SPAF
► Pivotal SPAF trials (AVERROES, RE-LY, and
ROCKET AF) focus on different subsets of
patients with AF: How will this affect riskspecific alignment of therapy for:
—Patients intolerant of, or unwilling to take
wafarin? (AVERROES, apixaban)
— Patients stratified to higher CHADS2 risk
scores? (ROCKET-AF, rivaroxaban)
— Patients stratified across multiple CHADS2
risk profiles (RE-LY, dabigatran)
Deciphering the Maze of RiskSpecific Interventions for SPAF
This Science-to-Strategy Summit will focus on
how best to translate landmark trials for SPAF
into the front lines of cardiovascular practice:
1.
Are cross trial comparisons possible? And what are the
implications for selecting new agents across the risk spectrum of
AF?
2.
How do we use CHADS2 risk scores, alone, or in combination with
other risk assessment tools? Is CHADS2 enough?
3.
Should goal be optimizing use of warfarin, using pharmacogenetic
and point-of-care approaches, or should it be risk-specific
alignment of new agents with carefully selected patient subsets
requiring SPAF?
4.
How will cost figure in to our decision-making?
New Paradigms in the
Science and Medicine of Heart Disease
Deciphering the Maze of RiskSpecific Interventions for Stroke
Prevention in Atrial Fibrillation
Aligning Therapy with Stroke Risk
Jonathan L. Halperin, MD
Professor of Medicine (Cardiology)
Mount Sinai School of Medicine
Director, Clinical Cardiology Services
The Zena and Michael A. Weiner
Cardiovascular Institute
The Marie-Josée and Henry R. Kravis Center
for Cardiovascular Health
Atrial Fibrillation
A Substantial Threat to the Brain
►
Affects
~4% of people aged >60 years
~9% of those aged >80 years
►
5%/year stroke rate
►
12%/year for those with prior stroke
►
$ billions annual cost for stroke care
►
AF-related strokes have worse outcomes
AF identifies millions of people with a
five-fold increased risk of stroke
Atrial Fibrillation
An Opportunity for Stroke Prevention
Affects ~1% of the population, ~10% of elderly
The number is rising
5%/year stroke rate
Is the rate falling?
12%/year for those with prior stroke
An opportunity for stroke prevention!
AF-related strokes have worse outcomes
Always costly, often horrible
$/€ billions annual cost for stroke care
Prevention means savings.
Anticoagulation in Atrial Fibrillation
Stroke Risk Reductions
Warfarin
Better
Control
Better
AFASAK
SPAF
BAATAF
CAFA
SPINAF
EAFT
Aggregate
100%
50%
Hart R, et al. Ann Intern Med 2007;146:857.
0
-50%
-100%
Anticoagulation in Atrial Fibrillation
The Standard of Care for Stroke Prevention
Warfarin
Better
Control
Better
Unblinded
AFASAK
SPAF
Unblinded
BAATAF
Unblinded
Terminated early
CAFA
Double-blind; Men only
SPINAF
2o prevention; Unblinded
EAFT
Aggregate
100%
50%
Hart R, et al. Ann Intern Med 2007;146:857.
0
-50%
-100%
Efficacy of Warfarin in Trials vs. Practice
Stroke Risk Reductions
Treatment
Better
Treatment
Worse
Warfarin vs.
Placebo/Control
6 Trials
n = 2,900
Warfarin vs.
No anticoagulation
Medicare cohort
n = 23,657
100%
Hart R, et al. Ann Intern Med 2007;146:857
Birman-Deych E. Stroke 2006; 37: 1070–1074
50%
0
-50%
Aspirin for Atrial Fibrillation
Stroke Risk Reductions
AFASAK I
SPAF I
EAFT
ESPS II
LASAF
UK-TIA
Aggregate
100%
50%
Aspirin
Better
Hart RG, et al. Ann Intern Med 2007; 147:590.
0
-50%
Aspirin
Worse
-100%
Aspirin for Atrial Fibrillation
Stroke Risk Reductions
AFASAK I
SPAF I
EAFT
ESPS II
LASAF
UK-TIA
Aggregate
100%
50%
Aspirin
Better
Hart RG, et al. Ann Intern Med 2007; 147:590.
0
-50%
Aspirin
Worse
-100%
Rates of Stroke or Systemic Embolism
in Anticoagulation Candidates
Warfarin or Aspirin
Placebo
Risk reduction
82%
Months
N Engl J Med 1990; 322: 863.
Stroke Risk in Atrial Fibrillation
Stroke Rate (% per year)
Untreated Control Groups of Randomized Trials
Age (years)
Atrial Fibrillation Investigators. Arch Intern Med 1994;154:1449.
Aspirin Effect Related to Age
SPAF-I Study
> 75 years
< 75 years
+ 150
Aspirin Better
0
Aspirin Worse
- 150
SPAF-II
Rates of Disabling Stroke
Ischemic
Hemorrhagic
6
4
Event
Rate
(%/year) 2
0
Aspirin
Warfarin
< 75 years
SPAF Investigators. Lancet 1994; 343: 687.
Aspirin
Warfarin
> 75 years
Rates of Disabling Stroke on Warfarin
Patients >75 Years Old
~1992
SPAF Investigators. Lancet 1994; 343: 687.
Mant J, et al. Lancet 2007; 370: 493.
~2006
Intracerebral Hemorrhage
The Most Feared Complication of Antithrombotic Therapy
► >10% of intracerebral hemorrhages (ICH)
occur in patients on antithrombotic therapy
► Aspirin increases the risk by ~40%
► Warfarin (INR 2–3) doubles the risk to 0.3–
0.6%/year
► ICH during anticoagulation is catastrophic
Hart RG, et al. Stroke 2005;36:1588
Risk Stratification in AF
Stroke Risk Factors
High-Risk Factors
►Mitral stenosis
►Prosthetic heart valve
►History of stroke or TIA
Singer DE, et al. Chest 2004;126:429S.
Fang MC, et al. Circulation 2005; 112: 1687.
Risk Stratification in AF
Stroke Risk Factors
High-Risk Factors
Moderate-Risk Factors
► Mitral stenosis
►Age >75 years
►Hypertension
►Diabetes mellitus
►Heart failure or ↓ LV
function
► Prosthetic heart valve
► History of stroke or TIA
Singer DE, et al. Chest 2008;133:546S.
Fang MC, et al. Circulation 2005; 112: 1687.
The CHADS2 Index
Stroke Risk Score for Atrial Fibrillation
Score (points)
Prevalence (%)*
Congestive Heart failure
Hypertension
Age >75 years
Diabetes mellitus
Stroke or TIA
1
1
1
1
2
32
65
28
18
10
Moderate-High risk
Low risk
>2
0-1
50-60
40-50
VanWalraven C, et al. Arch Intern Med 2003; 163:936.
* Nieuwlaat R, et al. (EuroHeart survey) Eur Heart J 2006 (E-published).
Nonvalvular Atrial Fibrillation
Stroke Rates Without Anticoagulation
According to Isolated Risk Factors
Prior
Hypertension Female
Age
Stroke/TIA > 75 years
Hart RG et al. Neurology 2007; 69: 546.
Diabetes Heart Failure
LVEF
CV Event Rates in Patients with AF
Related to CHADS2 Score
REACH Registry
Goto S, et al. Am Heart J 2008; 156: 855.
Risk Stratification in AF
Stroke Risk Factors
High-Risk Factors
Moderate-Risk Factors
► Mitral stenosis
►Age >75 years
►Hypertension
►Diabetes mellitus
►Heart failure or ↓ LV
function
► Prosthetic heart valve
► History of stroke or TIA
Less Validated Risk Factors
►
►
►
►
Age 65–75 years
Coronary artery disease
Female gender
Thyrotoxicosis
Singer DE, et al. Chest 2004;126:429S.
Fang MC, et al. Circulation 2005; 112: 1687.
Risk Stratification in AF
Stroke Risk Factors
High-Risk Factors
Moderate-Risk Factors
► Mitral stenosis
►Age >75 years
►Hypertension
►Diabetes mellitus
►Heart failure or ↓ LV
function
► Prosthetic heart valve
► History of stroke or TIA
Less Validated Risk Factors
►
►
►
►
Age 65–75 years
Coronary artery disease
Female gender
Thyrotoxicosis
Singer DE, et al. Chest 2004;126:429S.
Fang MC, et al. Circulation 2005; 112: 1687.
Dubious Factors
► Duration of AF
► Pattern of AF
- (persistent vs. paroxysmal)
► Left atrial diameter
The CHA2DS2VASc Index
Stroke Risk Score for Atrial Fibrillation
Weight (points)
Congestive heart failure or LVEF < 35%
Hypertension
Age >75 years
Diabetes mellitus
Stroke/TIA/systemic embolism
Vascular Disease (MI/PAD/Aortic plaque)
Age 65-74 years
Sex category (female)
1
1
2
1
2
1
1
1
Moderate-High risk
Low risk
>2
0-1
Lip GYH, Halperin JL. Am J Med 2010; 123: 484.
Risk Stratification and Anticoagulation
Stroke Reduction with Warfarin Instead of Aspirin
~100
~50
Event Rate (%/year)
>250
Number of
patients Neededto-treat
with
Warfarin vs.
Aspirin
to prevent
1 stroke/year
CHADS2 Score
AFASAK I, AFASAK II, ATHENS, BAFTA, EAFT, NASPEAF, PATAF, SIFA, SPAF II, SPAF III, WASPO
The CHADS2 Index
Stroke Risk in Patients with Atrial Fibrillation
Score
(points)
0
Approximate
Risk threshold for
Anticoagulation
Risk of Stroke
(%/year)
1.9
3%/year
1
2.8
2
3
4
5
6
4.0
5.9
8.5
12.5
18.2
Van Walraven C, et al. Arch Intern Med 2003; 163:936.
Go A, et al. JAMA 2003; 290: 2685.
Gage BF, et al. Circulation 2004; 110: 2287.
Antithrombotic Therapy for Atrial Fibrillation
ACC/AHA/ESC Guidelines 2006
Risk Category
No risk factors
CHADS2 = 0
One moderate risk factor
CHADS2 = 1
Any high risk factor or
>1 moderate risk factor
CHADS2 >2
or mitral stenosis
Prosthetic valve
Fuster V, et al. Eur Heart J 2006;27:1979.
Recommended Therapy
Aspirin, 81-325 mg qd
Aspirin, 81-325 mg/d or
Warfarin
Warfarin
(INR 2.0-3.0, target 2.5)
Warfarin
(INR 2.5-3.5, target 3.0)
Patient Selection for Anticoagulation
Additional Considerations
► Risk of bleeding
► Newly anticoagulated vs. established
therapy
► Availability of high-quality
anticoagulation management
program
► Patient preferences
Warfarin vs. Aspirin
Excess bleeds
(per 100 patients/year)
Excess Major Bleeding
11 trials, mean follow-up 1.8 years
The HAS-BLED Score
Risk Score for Predicting Bleeding in
Anticoagulated Patients with Atrial Fibrillation
Hypertension (>160 mmHg systolic)
Abnormal renal or hepatic function
Stroke
Bleeding history or anemia
Labile INR (TTR <60%)
Elderly (age >75 years)
Drugs (antiplatelet, NSAID) or alcohol
High risk (>4%/year)
Moderate risk (2-4%/year)
Low risk (<2%.year)
Weight (points)
1
1-2
1
1
1
1
1-2
>4
2-3
0-1
Pisters R, et al Chest 2010 (online) http://chestjournal.chestpubs.org/content/early/2010/03/18/chest.10-0134
The ACTIVE Trial
Clopidogrel + Aspirin
Atrial Fibrillation + Risk Factors
ACTIVE - W
Anticoagulation-eligible
VKA
(INR 2-3)
Clopidogrel
+ Aspirin
Open-label
Non-inferiority
n = 6,706
ACTIVE - A
OAC Contraindications or Unwilling
Aspirin
+ Placebo
Double-blind
Superiority
n = 7,554
Irbesartan, 300 mg/d vs. Placebo
n = 9,016
Risk Factors:
Age 75, hypertension, prior
stroke/TIA, LVEF<45%, PAD,
age 55-74 + CAD or diabetes
Clopidogrel
+ Aspirin
ACTIVE - I
Primary outcome: Stroke,
systemic embolism, MI or
cardiovascular death
The ACTIVE Trial
Clopidogrel + Aspirin
Atrial Fibrillation
ACTIVE – W
Anticoagulation-eligible
VKA
(INR 2-3)
Clopidogrel
+ Aspirin
Open-label
Non-inferiority
n = 6,706
+ Risk Factors
ACTIVE - A
OAC Contraindications or Unwilling
Aspirin
+ Placebo
Clopidogrel
+ Aspirin
Double-blind
Superiority
n = 7,554
Irbesartan, 300 mg/d vs. Placebo
n = 9,016
ACTIVE - I
Antithrombotic Therapy for Atrial Fibrillation
Stroke Risk Reductions
Warfarin
Better
Antiplatelet Rx
Better
ACTIVE-W
Anticoagulation vs.
Aspirin + Clopidogrel
n = 6,706
Anticoagulation vs.
Antiplatelet drugs
7 Trials
n = 4,232
100%
50%
Connolly S, et al. Lancet 2006; 367:1903.
Hart R, et al. Ann Intern Med 2007;146:857.
0
-50%
Antithrombotic Therapy for Atrial Fibrillation
Stroke Risk Reductions
Warfarin
Better
Antiplatelet Rx
Better
All patients
Warfarin vs.
Aspirin + Clopidogrel
Prior OAC
VKA-naïve
100%
Connolly S, et al. Lancet 2006; 367:1903.
50%
0
-50%
Major Hemorrhage in Relation to
Prior Anticoagulant Therapy
ACTIVE-W
“Starters”
“Switchers”
Interaction p=0.028
Yes
No
Anticoagulant Therapy at Entry
Connolly S, et al. Lancet 2006; 367:1903.
The ACTIVE Trial
Clopidogrel + Aspirin
Atrial Fibrillation
+ Risk Factors
ACTIVE – W
Anticoagulation-eligible
VKA
(INR 2-3)
Clopidogrel
+ Aspirin
ACTIVE - A
OAC Contraindications or Unwilling
Aspirin
+ Placebo
Open-label
Non-inferiority
n = 6,706
Double-blind
Superiority
n = 7,554
Irbesartan, 300 mg/d vs. Placebo
n = 9,016
Connolly SJ, et al. N Engl J Med 2009; 360:2066.
Clopidogrel
+ Aspirin
ACTIVE - I
ACTIVE-A
Reasons for Exclusion from Anticoagulation
•
•
•
•
Risk factor for bleeding*
23%
Physician judgment against
anticoagulation for patient
50%
Patient preference only
26%
Inability to comply with INR monitoring
Predisposition to falling or head trauma
Persistent hypertension >160/100 mmHg
Previous serious bleeding on VKA
• Severe alcohol abuse within 2 years
• Peptic ulcer disease
• Thrombocytopenia
• Chronic need for NSAID
Connolly SJ, et al. N Engl J Med 2009; 360:2066.
ACTIVE-A
Total Stroke Rates
Cumulative Incidence
0.15
28% RRR
408 (3.3%/year)
HR 0.72
(95% CI, 0.62–0.83)
p <0.001
Aspirin
0.10
296 (2.4%/year)
Clopidogrel + Aspirin
0.05
0.0
0
1
2
Years
Connolly SJ, et al. N Engl J Med 2009; 360:2066.
3
4
The ACTIVE Trials
Stroke Rates and Risk Reductions
Treatment
VKA
C+A
Aspirin
ACTIVE W
(Annual Rate)
1.4
2.4
~
ACTIVE A
(Annual Rate)
~
2.4
3.3
RRR
versus Aspirin
-58%
-28%
~
RRR
versus C+A
-42%
~
~
VKA = oral anticoagulant
C+A = clopidogrel + aspirin
Connolly SJ, et al. Lancet 2006; 367:1903.
Connolly SJ, et al. N Engl J Med 2009; 360:2066.
Apixaban vs. Aspirin: The AVERROES Trial
Primary and Secondary Endpoints
Outcomes
Apixaban
(n=2809)
Aspirin
(n=2791)
Relative risk
(95% CI)
Stroke or systemic
embolism
1.6
3.6
0.46 (0.33–0.64)
Stroke, embolism, MI, or
vascular death
4.1
6.2
0.66 (0.53–0.83)
MI
0.7
0.8
0.85 (0.48–1.50)
Vascular death
2.5
2.9
0.86 (0.64–1.16)
Cardiovascular
hospitalization
11.8
14.9
0.79 (0.68–0.91)
Total death
3.4
4.4
0.79 (0.62–1.02)
Connolly S. European Society of Cardiology, Stockholm, August 31, 2010
Apixaban vs. Aspirin: The AVERROES Trial
Bleeding Events
Outcomes
Apixaban
(n=2809)
Aspirin
(n=2791)
Relative risk
(95% CI)
Major bleeding
1.4
1.2
1.14 (0.74–1.75)
Fatal bleeding
0.1
0.1
0.84 (0.26–2.75)
Intracranial
0.4
0.3
1.09 (0.50–2.39)
Clinical relevant
non-major bleeding
3.0
2.6
1.18 (0.88–1.58)
Minor bleeding
5.2
4.1
1.27 (1.01–1.61)
Connolly S. European Society of Cardiology, Stockholm, August 31, 2010
RE-LY Trial
Primary Events
All Strokes and Systemic Embolic Events
Event Rate (%/year)
p <0.001 (Superiority)
p <0.001 (Noninferiority)
Connolly SJ et al. N Engl J Med 2009; 361: 1139.
RE-LY Trial
Hemorrhagic Stroke Events
Event Rate (%/year)
Intracerebral Hemorrhages
p <0.001
p <0.001
Connolly SJ et al. N Engl J Med 2009; 361: 1139.
Quality of Warfarin Control
International Normalized Ratio
SPORTIF-V
SPORTIF-III
ACTIVE-W
RE-LY
Warfarin control necessary
For efficacy noninferior to
Dabigatran 150 mg bid
Gage BF. N Engl J Med 2009; 361, 1200.
Oral Factor Xa Inhibitors
Ongoing Phase III Trials for Prevention of Stroke
and Systemic Embolism in Patients with AF
Trial
Acronym
Drug
Dose
Comparator
N
Risk
factors
ROCKET-AF
Rivaroxaban
20 mg*
qd
Warfarin
(INR 2-3)
14,000
≥2
ARISTOTLE
Apixaban
5 mg
bid
Warfarin
(INR 2-3)
15,000
≥1
ENGAGE-AF
Edoxaban
30 mg bid
60 mg* qd
Warfarin
(INR 2-3)
~20,000 ≥ 2
* Adjusted based on renal function
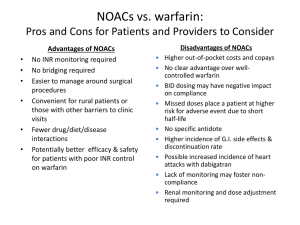
Antithrombotic Therapy for Atrial Fibrillation
The Future of Risk-Stratified Treatment?
Risk Category
No risk factors
CHADS2 = 0
One moderate risk factor
CHADS2 = 1
Recommended Therapy
Aspirin, 81-325 mg qd
Warfarin
or clopidogrel + aspirin
or Factor IIa or Xa
inhibitor
Any high risk factor or
>1 moderate risk factor
CHADS2 >2
Warfarin
or Factor IIa or Xa
inhibitor
Mitral stenosis or
Prosthetic valve
Warfarin
(? higher intensity)
Fuster V, et al. Eur Heart J 2006;27:1979.
Alternatives to Anticoagulation
Atrial Fibrillation
Current approaches
Restoration and maintenance of sinus rhythm
• Antiarrhythmic drug therapy
• Catheter ablation
• Maze operation
Emerging (investigational) approaches
Obliteration of the left atrial appendage
• Trans-catheter occluding devices
• Thoracoscopic epicardial plication
• Amputation
Strokes after Conversion to NSR
Rate vs. Rhythm Control Trials
Rate
control
Rhythm
control
RR
(95% CI)
p
4,917
5.7%
7.3%
1.28 (0.95-1.72)
0.12
RACE
522
5.5%
7.9%
1.44 (0.75-2.78)
0.44
STAF
266
1.0%
3.0%
3.01 (0.35-25.3)
0.52
PIAF
252
0.8%
0.8%
1.02 (0.73-2.16)
0.49
Total
5,957
5.0%
6.5%
1.28 (0.98-1.66)
0.08
Trial
AFFIRM
n
Verheugt F, et al. J Am Coll Cardiol 2003;41(suppl):130A.
AFFIRM Trial
Stroke Rates
►74% of all strokes were proven ischemic
• 44% occurred after stopping warfarin
• 28% in patients taking warfarin with INR <2.0
• 42% occurred during documented AF
Wyse AG, et al. N Engl J Med 2002; 347: 1825.
ATHENA Trial
Dronedarone vs. Placebo in Patients with AF
Stroke Rates
Event
Placebo
(%/y)
(Secondary Analysis)
Dronedarone
HR
(%/y)
(95% CI)
p
Stroke
1.8
1.2
0.66
0.027
Ischemic stroke
1.3
0.9
0.68
0.081
Stroke or TIA
2.3
1.6
0.70
0.031
Fatal stroke
0.5
0.4
0.67
0.247
Stroke, ACS or CV
death
5.5
3.8
0.68
<0.001
Connolly S, et al Circulation 2009; 120:1174.
Cardiovascular Outcomes in the
ATHENA Trial
Rates of Stroke, ACS or Cardiovascular Death
p < 0.001
p = 0.538
Percutaneous LAA Occlusion
The WATCHMAN® Device
Syed T, Halperin JL. Nature Clin Prac Cardiovasc Med 2007; 4:428
Holmes DR, et al. Lancet 2009; 374: 534
PROTECT-AF Trial
All Strokes (ITT Analysis)
Device
Events Total
Superiority
pt-yr
582.9
Rate
Events
(95% CI)
2.6
(1.5, 4.1)
Total
Rate
pt-yr (95% CI)
11
318.1
Event-free probability
15
Posterior
probabilities
Control
RR
Non-
(95% CI) inferiority
3.5
0.74
0.998 0.731
(1.7, 5.7) (0.36, 1.76)
WATCHMAN
Control
Days
Holmes D, et al. ACC & i2 Summit, Orlando, FL, March 28, 2009
Atrial Fibrillation and Thromboembolism
The Next Challenges
►
Better risk-stratification that balances stroke
and bleeding
►
Noninvasive methods to predict events and
guide therapy
►
Safe treatments for the highest risk patients
►
Achieving and confirming successful rhythm
control over time
►
Targeted AF prevention
From Fermented Sweet Clover
to Molecular Targeting of Coagulation
The Promise of New Approaches
The Goal:
To bring effective therapy to many
more patients and prevent thousands of
strokes.
New Paradigms in the
Science and Medicine of Heart Disease
FROM RISK TO REMEDY:
Role of Thrombin and Factor Xa
Inhibitors for Stroke Prevention in AF
Jeffrey I. Weitz, MD, FRCP(C), FACP
Professor of Medicine and Biochemistry
McMaster University
Canada Research Chair in Thrombosis
Heart & Stroke Foundation/ J.F. Mustard Chair
in Cardiovascular Research
Overview
► Risk of stroke in AF
► Choice of antithrombotic drugs
► Which drug for which patient
Atrial Fibrillation
Major Risk Factor for Stroke
► Independent risk factor for stroke
- increases risk of stroke 5-fold
► Accounts for 15-20% of all strokes
► Patients with paroxysmal and
sustained AF have same risk of
stroke
Wolf PA, et al. Stroke 1991
Savelieva I, et al. Ann Med 2007
Singer DE, et al. Chest 2008
AF=atrial fibrillation
Antithrombotic Drug Choices
for Stroke Prevention
►Antiplatelet agents
• ASA
• ASA plus clopidogrel
►Anticoagulants
Which Drug for Which Patient?
CHADS2 Score
Agent
0
ASA or nothing
1
ASA or anticoagulation
>2
Anticoagulation
ACCP 2008
Are Patients With CHADS2 of 0-1 Really
Low Risk? Role of CHADS2-Vasc Score
Risk Factor
Score
CHF or LV dysfunction
1
Hypertension
1
Age > 75
2
Diabetes
1
Stroke or TIA
2
Vascular disease
1
Age 65-74
1
Sex category (female)
1
Pamukcu et al. Age and ageing 2010
CHADS2-Vasc Score and
Antithrombotic Therapy
ESC guidelines 2010
Score
Therapy
0
ASA or nothing
1
ASA or anticoagulation
>2
Anticoagulation
Anticoagulant Choices for
Stroke Prevention
► Warfarin
► New oral anticoagulants
• Dabigatran
• Rivaroxaban
• Apixaban
• Edoxaban
Limitations of Warfarin
Limitation
Consequence
Slow onset of action
Overlap with a parenteral
anticoagulant
Genetic variation in
metabolism
Variable dose requirements
Multiple food and drug
interactions
Frequent coagulation
monitoring
Narrow therapeutic index
Frequent coagulation
monitoring
Comparison of Features of New
Anticoagulants with Those of Warfarin
Features
Warfarin
New Agents
Onset
Slow
Rapid
Dosing
Variable
Fixed
Yes
No
Many
Few
Yes
No
Half-life
Long
Short
Antidote
Yes
No
Food effect
Drug interactions
Monitoring
The Current Landscape
How Do the New Oral
Anticoagulants Compare
with Warfarin?
Stroke or Systemic Embolism
Non-inferiority Superiority
p-value
p-value
Dabigatran 110 vs. Warfarin
<0.001
0.34
Dabigatran 150 vs. Warfarin
<0.001
<0.001
Margin = 1.46
0.50
0.75
Dabigatran better
Connolly et al., NEJM, 2009
HR
1.00
1.25
(95% CI)
1.50
Warfarin better
Annual Rates of Bleeding
D
110mg
D
150mg
Warfarin
6015
6078
6022
RR
95% CI
p
RR
95% CI
p
Total
14.6%
16.4%
18.2%
0.78
0.74-0.83
<0.001
0.91
0.86-0.97
0.002
Major
2.7 %
3.1 %
3.4 %
0.80
0.69-0.93
0.003
0.93
0.81-1.07
0.31
LifeThreatening
1.2 %
1.5 %
1.8 %
0.68
0.55-0.83
<0.001
0.81
0.66-0.99
0.04
Gastrointestinal
1.1 %
1.5 %
1.0 %
1.10
0.86-1.41
0.43
1.50
1.19-1.89
<0.001
N
Connolly et al., NEJM, 2009
D 110mg vs.
Warfarin
D 150mg vs.
Warfarin
Intracranial Bleeding Rates
RR 0.31 (95% CI: 0.20–0.47)
p<0.001 (sup)
RR 0.40 (95% CI: 0.27–0.60)
p<0.001 (sup)
Number of events
0,74 %
RRR
60%
RRR
69%
0,30 %
0,23 %
Connolly et al., NEJM, 2009
Which Patients are Candidates
for Dabigatran?
► Higher dose dabigatran regimen more
effective than warfarin in all CHADS2
categories
Stroke and Systemic Embolism
D110 vs. warfarin
D150 vs. warfarin
P = 0.44
P = 0.82
Annual rate, %
CHADS2 D110 D150 WARFARIN
0-1
1.06
0.65
1.05
2
1.43
0.84
1.38
3-6
2.12
1.88
2.68
0.50
1.00
1.50
Dabigatran
Warfarin
better
better
Oldgren et al., JACC,
2010
0.50
1.00
Dabigatran
better
1.50
Warfarin
better
What Have We Learned About
Dabigatran Versus Warfarin?
► Benefits of dabigatran over warfarin
apparent at all levels of TTR
► Benefits of dabigatran greatest in
centers with lowest TTR
RRR in Stroke or Systemic Embolism with
Dabigatran Versus Warfarin by TTR by Center
TTR (%)
Dabigatran 110 mg
Dabigatran 150 mg
< 57
1.0 (0.68-1.45)
0.6 (0.37-0.88)
57-65
0.8 (0.56-1.17)
0.5 (0.33-0.77)
66-73
0.9 (0.58-1.36)
0.7 (0.44-1.09)
>73
0.9 (0.59-1.45)
0.9 (0.61-1.48)
Yes
No
Half-life
Long
Short
Antidote
Yes
No
Monitoring
Wallentin et al, Lancet, 2010
What About Dabigatran in
the Elderly?
► Lower risk of stroke and intracranial bleeding
► Higher risk of extracranial (mostly GI) bleeding
Stroke or Systemic Embolism
Dabigatran110 vs. warfarin
Dabigatran150 vs. warfarin
Rate(% per year)
Age <65
D110 D150WAR
1.48 0.69 1.35
Age 65-74 1.26
0.98 1.43
1.87
1.43 2.1
Age ≥75
CrCl 30-50 2.26 1.33 2.65
CrCl 51-80 1.65
1.24 1.76
CrCl >80 0.92 0.72 1
0.50 1.00 1.50
Dabigatran better
Healey et al., JACC, 2010
0.50 1.00 1.50
Warfarin better Dabigatran better
Warfarin better
Hemorrhagic Stroke
Dabigatran110 vs. warfarin
Rate(% per year)
Dabigatran150 vs. warfarin
D110 D150 WAR
Age <65
0.05 0.05 0.38
Age 65-74 0.08 0.08 0.31
Age ≥75
0.2
0.15 0.47
CrCl 30-50 0.26 0.12 0.58
CrCl 51-80 0.12 0.09 0.47
CrCl >80
0.03 0.08 0.13
0
0.5
Dabigatran better
Healey et al., JACC, 2010
1.0
1.5
2.0
0
0.5
1.0
1.5
2.0
Warfarin better Dabigatran better Warfarin better
Major Bleeding
Dabigatran110 vs. warfarin
Dabigatran150 vs. warfarin
Rate(% per year)
D110 D150WAR
Age <65
0.76 0.79 2.32
Age 65-74 2.12 2.45 3.08
Age ≥75
4.21 4.81 4.09
CrCl 30-50 5.07 4.85 5.17
CrCl 51-80 2.62 3.04 3.44
CrCl >80
1.36 1.88 2.18
0.50 1.00 1.50
Dabigatran better
Healey et al., JACC, 2010
0.50 1.00 1.50
Warfarin better Dabigatran better
Warfarin better
Which Dabigatran Dose for
Which Patient?
CrCl (ml/min)
Dose
>30
150 mg BID
15-30
75 mg BID
< 15
Contraindicated
What About The Elderly?
Health Canada approved the 110 mg
dose for patients > 80 years of age
Who is Not a Candidate for Dabigatran?
► Stable on warfarin?
► CrCl less than 15 ml/min
► Severe hepatic dysfunction
► High risk of GI bleeding?
► Mechanical valve
The Current Landscape
Practical Issues in
Dabigatran Management
Switching from Warfarin
to Dabigatran
Start dabigatran when INR ≤ 2.0
Assessment of Dabigatran
Activity
►Normal aPTT and/or thrombin time indicates
absent activity
►Dabigatran calibrators will be available
Unanswered Questions
With Dabigatran
► Will dyspepsia lead to discontinuation?
► How will we manage patients with a history of
GI bleeding?
► Will short half-life obviate need for an antidote?
What About Other Agents?
Agent
Trial
Rivaroxaban
ROCKET
Apixaban
ARISTOTLE
Edoxaban
ENGAGE
ROCKET-AF: LATE-BREAKING
Placeholder for ROCKET-AF Trial
Results
Opportunities for Oral Factor Xa Inhibitors
► Once daily dosing
► No dyspepsia
► No excess GI bleeding
► Better benefit-to-risk profile in elderly?
Challenges for New Oral
Anticoagulants?
►Costs will be high – who will pay?
►How will we assess compliance?
►How will we treat bleeds?
Conclusions
► New oral anticoagulants are poised to replace
warfarin for stroke prevention in AF
► Dabigatran is the first, but others will soon
follow
► How the new agents compare with each other
remains to be determined
New Paradigms in the
Science and Medicine of Heart Disease
The Evolving Paradigm of
Warfarin-Based Therapy: Still
Relevant? And for How Long?
David A. Garcia, MD
Associate Professor, Division of General Internal Medicine
University of New Mexico
Co-Director, University of New Mexico
Anticoagulation Management Service
President, Anticoagulation Forum
Long-Term Oral Anticoagulation
The State of the Art
Warfarin Protects Against Stroke
Adjusted-Dose Warfarin Compared With Placebo
Hart, et al. Ann Intern Med. 2007 Jun 19;146(12):857-67.
Warfarin
first used
AC management clinics
become increasingly prevalent
POC testing, PST,
dosing algorithms,
software programs,
better understanding of
genetics and drug interactions
WHO endorses INR
1950’s
1983
1991
1999
Efficacy of VKA
to prevent AF-related stroke
demonstrated
Warfarin
Mechanism of Action
Vitamin K
VII
Vitamin K
Utilization Impaired
CYP450
Warfarin
IX Synthesis of
X
II
Dysfunctional
Coagulation
Factors
Slight genetic variation can produce
significant differences in dose-response
relationship
Warfarin
Mechanism of Action
Decarboxylated zymogen
Vitamin KH2
Vitamin K
reductase
NADPH
Slight genetic
variation can
produce significant differences
in dose-response relationship
Carboxylated zymogen
Vitamin K Epoxide
Vitamin K
X
Vitamin K epoxide
reductase
Coagulation Pathway
Initiation
Vlla/TF
X
IX
Propagation
IXa
VllIa
Xa
II
Va
IIa (Thrombin)
Fibrin Formation
Fibrinogen
Fibrin
Limitations of Warfarin (VKA)
Anticoag. Clinics
Complicates Management
Of:
• Bleeding patient
• Patient with High INR
Narrow
Therapeutic
Index & Drug/Diet
Interactions
Long
Half-Life
Slow
Onset of
Action
Requires frequent
Monitoring
? Genotype testing
Heparin “overlap”
often necessary
Periprocedural Anticoagulation Difficult
Is VKA (Warfarin) Therapy Getting
Safer and more “User-friendly”?
►
Widespread use of the INR
►
Anticoagulation management services
►
Patient self-testing
►
Pharmacogenomics
►
Reversal strategies
Is VKA (Warfarin) Therapy Getting Safer
and more “User-friendly”?
Does “time in therapeutic
range” matter?
Events per 100 Patient-Years According to
International Normalized Ratio Control
Poor
Control
Event
Moderate
Control
Good
Control
(n =
1190)
P Value
(Poor
vs Good)
(n =
1207)
P Value
(Moderate
vs Poor)
(n =
1190)
P Value
(Good vs
Moderate)
Stroke or systemic
embolism
2.1
0.02
1.34
0.09
1.07
0.48
Death, all cause
4.2
< .01
1.84
< .01
1.69
0.74
Death, stroke, or
systemic embolism
5.98
< .01
3.01
< .01
2.76
0.67
Major bleeding
3.85
< .01
1.96
< .01
1.58
0.38
White H et al. Arch Intern Med. 2007.167:239-245
Increased “Time-in-Range” =
Better Outcomes
“Stable”
patients
N= 2504
Comparator
group
N=3569
p value
Deceased, %
0.4
1.6
< .001
AC-related death, %
0.04
0.1
.411
AC-related thrombosis, %
0.4
0.7
0.1
AC-related bleeding, %
0.8
2.8
< .001
AC-related bleeding or
thrombosis, %
1.1
3.6
< .001
Outcome
Witt et al. Blood 2009. 114: 952-956.
Dabigatran vs. Warfarin, Stratified by
Center-Based Time-in-Range
Time to stroke or systemic embolism
Wallentin et al. Lancet 2010. 376:975-83
% of patients
Anticoagulation Management Service
Can Increase Time-in-Range
% time in range
Witt et al. Chest 2005.
■Clinical Pharmacy AMS
■Control (Usual Care)
Anticoagulation Management Services
►
PROS
●
●
►
Increase TTR
Probably improve outcomes
CONS
●
●
Resource-intensive
Only available to a minority of patients in many
countries (including the United States)
Patient self-testing may reduce risk
of thromboembolic events
Heneghan et al. Lancet. 2006 Feb 4;367(9508):404-11.
Self-testing vs. Clinic Testing:
A Randomized Trial
Time to stroke, major bleed or death
Matchar et al. NEJM 2010 363:1608-1620
Patient Self-testing
►
Not feasible for all warfarin-treated
patients
►
Uptake in U.S. also limited by low CMS
reimbursement for physician oversight
►
Recently published RCT suggests no better
than “high-quality” AC-clinic based
management
Knowledge of Genotype Allows
Better Dose Prediction
Gage B. NEJM 2005.
Anderson et al RCT (n=200)
►
Only published high-quality RCT
►
Incorporated both CYP2C9 and VKORC1 genotypes
PG (%)
Std (%)
p
Out-of-range INR
31
33
0.72
Therapeutic INR by day 5
70
68
0.85
Serious adverse events
4
5
0.71
Anderson et al, Circulation, 2007; 116: 2563-70.
Meta-analysis: % Time Therapeutic
Kangelaris, JGIM, 2009; 24(5): 656-64.
Pharmacogenetic Testing
►
Promising science but…
►
Clinical benefit still unproven
Reversal Strategies
4-factor prothrombin
complex concentrate
administered
Preston et al. British Journal of Haematology 2002. 116: 619–624
Warfarin Reversal
►
PO/IV vitamin K
●
►
Fresh frozen plasma
●
●
►
Effective but INR decrease not immediate
Each unit contains limited amount of vitamin-K
dependent clotting proteins
Significant volume challenge; infection risk
4-factor PCC
●
●
●
Not available in U.S.
? Cost
? Risk of thrombosis
Conclusions
►
Although warfarin treatment is safer and more
practical than it was 10-15 years ago, there is
certainly room for further improvement.
►
The new agents in development may
●
●
Eliminate some of the challenges unique to warfarin
Allow patients for whom warfarin is not feasible to receive
highly effective anti-thrombotic therapy
Questions Regarding the New
Oral Anticoagulants
►
Do they represent a significant improvement for
patients who have been taking warfarin with
consistently therapeutic INR values for
months/years?
►
Will the elimination of regular INR measurement
reduce compliance?
►
How will their cost compare to current costs
(including INR monitoring, dose adjustment,
etc.)?
►
Which (if any) will be available to patients with
significantly impaired renal function?
How do I Convert a Patient from
Warfarin to Dabigatran and Vice Versa?
► Warfarin to dabigatran: discontinue warfarin and start
dabigatran when the international normalized ratio
(INR) is below 2.0.
► Dabigatran to warfarin:
• CrCl >50 mL/min, start warfarin 3 days before discontinuing dabigatran.
• CrCl 31-50 mL/min, start warfarin 2 days before discontinuing dabigatran.
• CrCl 15-30 mL/min, start warfarin 1 day before discontinuing dabigatran.
• CrCl <15 mL/min, no recommendations can be made.
Because dabigatran can contribute to an elevated INR, the INR
will better reflect warfarin’s effect after dabigatran has been
stopped for at least 2 days.
Dabigatran prescribing information 2010.
What should I do if my patient has
an ischemic stroke on dabigatran?
►
Consider:
●
●
●
Is the patient compliant with dabigatran? Check
aPTT – if dose taken within past 12 hours, it
should be prolonged.
If the stroke is not “cardiogenic”, consider adding
anti-platelet therapy.
Convert dabigatran to warfarin (target INR 2-3 or
higher?).
What should I do if my patient on
dabigatran is bleeding?
►
Volume support – maintain good renal function.
►
Anatomic interventions
●
●
►
Animal models*
●
●
►
Direct compression
Arterial embolization
Prothrombin complex concentrate (e.g. F.E.I.B.A.)?
Recombinant factor VIIa?
Hemodialysis
* Wienen W, J Thromb Haemost 2008; 3(Suppl. 1): P1703.
How do I Prepare a Patient on
Dabigatran for Elective Surgery?
►
If possible, discontinue dabigatran 1 to 2 days
(CrCl ≥50 mL/min) prior
●
►
or 3 to 5 days (CrCl <50 mL/min)
Consider longer times for patients undergoing
major surgery, spinal puncture, or placement of
a spinal or epidural catheter or port, in whom
complete hemostasis may be required
Dabigatran prescribing information 2010.
New Paradigms in the
Science and Medicine of Heart Disease
So What Now?
Applying Remedies to Risk Groups in
the Real World Setting for SPAF
Case Study Analysis with Audience Response System
(ARS): Focus on Risk-Specific Alignment of
Thromboprotective in Patients with AF
Elaine M. Hylek, MD, MPH
Associate Professor of Medicine
Department of Medicine
Boston University Medical Center
Boston, MA
Atrial Fibrillation Case Study
Atrial Fibrillation Case Study
► An 82-year-old man with
hypertension and diabetes has
permanent atrial fibrillation
► He has a history of spinal stenosis
and walks with a walker
Atrial Fibrillation Case Study
Question 1: Which regimen would you
prescribe for prophylaxis against
thromboembolism?
a. Warfarin (INR 2.0-3.0)
b. Warfarin (INR 1.5-2.0)
c. Aspirin, 81 mg daily
d. Aspirin, 81 mg + clopidogrel, 75 mg daily
e. An oral Factor Xa or direct thrombin
inhibitor
Atrial Fibrillation Case Study
Assessment of Thromboembolic Risk
Score
(points)
Risk of Stroke
(%/year)
0
1.9%
1
2.8%
2
4.0%
3
5.9%
4
8.5%
5
12.5%
6
18.2%
Van Walraven C, et al. Arch Intern Med 2003; 163: 936.
Go A, et al. JAMA 2003; 290: 2685.
Gage BF, et al. Circulation 2004; 110: 2287.
Atrial Fibrillation Case Study
Question 2: What if you learn that he has tripped
and fallen twice in the past two years?
a. Warfarin (INR 2.0-3.0)
b. Warfarin (INR 1.5-2.0)
c. Aspirin, 81 mg daily
d. Aspirin, 81 mg + clopidogrel, 75 mg daily
e. An oral Factor Xa or direct thrombin
inhibitor
Atrial Fibrillation Case Study
Question 3: In this patient with a history of tripping
you would treat with:
a. Warfarin (INR 2.0-3.0)
b. Warfarin (INR 1.5-2.0)
c. Dabigatran 150 mg P.O. B.I.D.
d. Aspirin, 81 mg + clopidogrel, 75 mg daily
e. Rivaroxaban 20 mg PO once-daily
Atrial Fibrillation Case Study
Anticoagulation in Patients at Risk of Falls
Atrial Fibrillation Case Study
Anticoagulation in Patients at Risk of Falls
“…persons taking warfarin must fall about
295 (535/1.81) times in 1 year for
warfarin not to be the optimal therapy…”
Atrial Fibrillation Case Study
ICH in Patients with AF Prone to Falls
Hazard ratios of independent
predictors of intracranial hemorrhage
Hazard ratio
(95% CI)
P value
High-risk for falls
1.9 (1.03-2.9)
0.002
Prior stroke
2.2 (1.7-2.8)
<0.0001
Prior bleed
1.8 (1.4-2.4)
<0.0001
Neuropsychiatric
impairment
1.4 (1.0-1.9)
0.055
Factor
► The risk of ICH was 2.8%/year in
patients at high risk of falls and 1.1 in
other patients.
► Warfarin was associated with an
increased risk of mortality among
those with ICH (30 day mortality = 52
vs. 34%, p = 0.007).
Gage BF, et al. Am J Med 2005; 118:612.
Atrial Fibrillation Case Study
Outcomes in Patients with AF Prone to Falls
Hazard ratio of warfarin for composite outcome—out-ofhospital death or hospitalization for stroke, MI, or
hemorrhage—in 1245 patients at high risk for falls
CHADS 2
score
Hazard ratio
(95% CI)
P value
Recommended
antithrombotic
therapy
0-1
0.98 (0.56, 1.72)
0.94
Aspirin or nil
2-6
0.75 (0.61, 0.91)
0.004
Anticoagulant
Gage BF, et al. Am J Med 2005; 118:612.
Summary of Case Study
► The risk of intracranial hemorrhage is increased
in patients who fall.
► The use of oral anticoagulation does not predict
ICH, but mortality is higher among patients on
anticoagulants who develop ICH.
► The risk of mortality due to ICH is offset by the
reduction in ischemic events achieved with
anticoagulation in elderly patients with AF at
high risk of thromboembolism.
► Better risk-stratification instruments are needed.
Atrial Fibrillation Case Study
Atrial Fibrillation Patient Case Study
► 85-year-old female with AF, HTN, HF, prior
TIA, osteoarthritis and prior diverticular GIB
six months ago, on warfarin, who presents to
the ED with complaints of SOB for several
days and black stools.
► Medications: atenolol, lisinopril, lasix,
warfarin, ASA
► Most recent INR 3 weeks ago = 3.1
Atrial Fibrillation Case Study
Question 1: This patient’s estimated stroke risk
per year without warfarin is:
a) 5%
b) 12%
c) 20%
d) None of the above
Physical Exam and Pertinent Data
Exam: Afebrile, HR 110-130, BP 154/90
Lungs-bibasilar rales
COR-irreg irreg
ABD-nontender
Guaiac +
ECG:
AF with rapid VR
CXR:
Mild pulmonary edema
Labs:
Hct=21, INR=8.0, Troponin -
Atrial Fibrillation Case Study
Question 2: The most appropriate management
strategy for this patient would be to:
a) Stop both aspirin and warfarin – Resume aspirin only
in one week
b) Stop both aspirin and warfarin – Resume warfain
c) Stop both aspirin and warfarin – Resume both
warfarin and aspirin in one week
d) Stop both aspirin and warfarin permanently
Atrial Fibrillation Case Study
Question 3: The patient’s bleeding episode resolves,
she is started back on warfarin, and she returns six
months later with an hematocrit of 35 (her baseline).
Her INR is 3.7. At this point you would:
a) Stop warfarin and put the patient on clopidogrel and
aspirin
b) Adjust the warfarin to achieve an INR of 2.0 - 3.0
c) Transition patient to dabigatran 150 mg PO BID
d)
Transition patient to rivaroxaban 20 mg PO QD
e) Start aspirin only
f) Stop all anticoagulation
Atrial Fibrillation Case Study
Atrial Fibrillation Case Study
► Mrs. A. is a 78-year-old woman who is taking
warfarin for stroke prevention on the background
of atrial fibrillation. She also takes ASA 81 mg
daily.
► Her risk factors for stroke include hypertension
and type II diabetes mellitus. Her INR control has
been erratic with values ranging from 1.5 to 6.8.
► For the past two weeks, she has had intermittent
nosebleeds lasting 5 to 20 minutes. She is
anxious to stop warfarin.
Atrial Fibrillation Case Study
Question 1: What is the best approach for this
patient?
(a) Stop the warfarin and the ASA
(b) Stop the ASA, but continue warfarin
(c) Perform CYP2C9 and VKORC1 genotyping to
better identify an appropriate warfarin dose
(d) Stop the warfarin and add clopidogrel 75 mg daily
(e) Continue warfarin and ASA, but monitor the INR
more frequently
Atrial Fibrillation Case Study
► The ASA was stopped, but Mrs. A. still
complains of nosebleeds.
► Despite weekly monitoring, her INR
continues to range from 1.8 to 4.8.
► A calculated creatinine clearance is 45
ml/min.
Atrial Fibrillation Case Study
Question 2: What would you likely do at this point?
(a) Continue on warfarin
(b) Continue on warfarin, but add low-dose vitamin K
(c) Switch from warfarin to dabigatran 150 mg BID
(d) Switch from warfarin to dabigatran 75 mg BID
(e) Switch from warfarin to ASA and clopidogrel
(f) Switch from warfarin to rivaroxaban 20 mg QD
(g) Switch from warfarin to rivaroxaban 15 mg QD
New Frontiers in Atrial Fibrillation
ATRIAL FIBRILLATION
Deciphering the Maze:
What Have the Trials Told Us? What Will
SPAF Look Like for Cardiologists at the
Front Lines of Clinical Practice?
Samuel Z. Goldhaber, MD
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
Deciphering the Maze:
Anticoagulation Efficacy and Safety
►
Efficacy: Dabigatran, the first
approved novel oral anticoagulant,
is superior to warfarin for SPAF:
34% reduction in stroke
►
Safety: Dabigatran causes less
intracerebral hemorrhage than
warfarin: 60% reduction in ICH
Efficacy: A Driving Force
for FDA Approval
► The 150-mg dose of dabigatran was
superior to warfarin (relative risk, 0.66;
95% confidence interval [CI], 0.53 to
0.82; P<0.001)
► But the 110-mg dose was not (relative
risk, 0.91; 95% CI, 0.74 to 1.11; P =
0.34).
Safety: A Driving Force for
FDA Approval
Warfarin versus dabigatran 150 mg rates of:
►Life-threatening bleeding (1.8% vs. 1.2%)
►Intracranial bleeding (0.7% vs. 0.2%)
►Major or minor bleeding (18.2% vs. 16.4%)
Deciphering the Maze:
“Cost-effectiveness”
Cost: Must take into account the costs
of caring long-term for debilitated
thromboembolic stroke patients and the
costs of caring for intracranial
hemorrhage when doing a “costeffectiveness” analysis of dabigatran
versus warfarin.
However, we continue to have mostly “silo budgeting.”
Drug Costs per Day:
Dabigatran versus Warfarin
Dabigatran
$6.75 per day
Warfarin
$0.75 per day (average)
Warfarin INR Testing
$3.00 per day
Warfarin PharmD/RN
$1.00 per day
Difference in Cost
$2.00 per day
Incremental Effectiveness:
Dabigatran versus Warfarin
Dabigatran:
► 0.5% lower annual mortality (p=0.051)
► 0.6% lower annual stroke (p<0.001)
► 0.4% lower annual ICH (p<0.001)
Versus $ 730/year additional drug cost
What Happens When You Account for Cost Of
Complications (Stroke, Major Bleeding and MI)
►
Treating 200 patients with dabigatran for a year costs an
additional $186,00 over warfarin
►
Among 200 treated patients, there is a cost reduction of
$112,000 due to 1.12 fewer stroke cases with dabigatran
►
Among 200 treated patients, there is a cost reduction of
$4,800 due to 0.6 fewer major bleeds
►
Among 200 treated patients, there is a cost increase of
$2,800 due to 0.4 MI cases
• The total additional cost of dabigatran treatment inclusive of
complications in 200 patients is $81,600
• The cost per year of life saved assuming 6.75 years of
survival is $12,089
Freeman, JV et. Al., AIM, 2010, Nov 3, Cost-effectiveness of dabigatran vs warfarin for SPAF
What About Warfarin Compared to
New Oral Anticoagulants?
Warfarin: Reinventing itself with rapid
turnaround genetic testing, point of care
testing, specialized and centralized clinics.
The net result is more effective and safer
therapy. How or whether these
improvements will change approach to new
agents remains to be seen.
ESC AF Guidelines: September, 2010
ESC AF Guidelines: September, 2010
New ESC Guidelines for AF
► OACs, such as a VKA, should be adjusted to an intensity
range of INR 2.0–3.0 (target 2.5).
► New OAC drugs [DTIs and factor Xa inhibitors] which may
be viable alternatives to a VKA, may ultimately be
considered.
—Where oral anticoagulation is appropriate therapy, dabigatran may
be considered, as an alternative to adjusted dose VKA therapy . . .
dabigatran 150 mg b.i.d. may be considered, in view of the improved
efficacy in the prevention of stroke and systemic embolism (but
lower rates of intracranial haemorrhage and similar rates of major
bleeding events, when compared with warfarin)
Europace (2010) 12, 1360–1420 doi:10.1093/europace/euq350, p. 16
Next Steps: Information to Application
1.
Hospital formulary committee evaluations
2.
Reevaluate whether established AF patients meet the
current guidelines for anticoagulation
3.
Reevaluate rate vs. rhythm control
4.
Determine whether to transition to dabigatran or continue
warfarin; and analyze new trials and data being aware of
pitfalls of cross-trial comparisons
5.
Address risk factors that predispose to AF, thereby
preventing AF if possible
6.
Deciphering the maze of risk-specific interventions for SPAF
will challenge us for years to come