MULTIPLE MYELOMA AND PLASMACYTOMA
advertisement

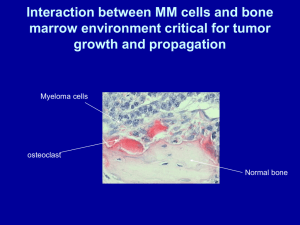
DR KARANU JK Most It common primary malignancy of bone is a plasma cell disorder monoclonal neoplasms related to each other by virtue of their development from common progenitors in the B lymphocyte lineage Thoughts that plamacytoma is simply an early, isolated form of multiple myeloma There are two important variants of myeloma, solitary bone plasmacytoma extramedullary plasmacytoma Solitary bone plasmacytoma is a single lytic bone lesion without marrow plasmacytosis Extramedullary plasmacytomas usually involve the submuscosal lymphoid tissue of the nasopharynx or paranasal sinuses without marrow plasmacytosis. The cause of myeloma is not known Incresed frequency in those exposed to radiation Seen more frequently among farmers, wood workers, leather workers and those exposed to petroleum products Chromosomal alterations identified: 13q14 deletions 17p13 deletions 11q abnormalities Common translocations t(11;14)(q13;q32) and t(4;14)(p16;q32) Overexpression of myc or ras genes has been noted in some cases Mutations in p53 and Rb1 have also been described No common molecular pathogenesis has yet emerged Represents more than 40% of primary bone cancers Peak 2:1 incidence is in 5th to 7th decades male predominance Blacks have nearly twice the incidence of whites. Yearly incidence is around 4 per 100000 Knh multiple myeloma 337 cases in 5 years, average of 67 per year Plasmacytoma 16 cases in 5 years, average of 3 per year Multiple myeloma cells bind via cell surface adhesion molecules to bone marrow stromal cells and extracellular matrix. This triggers multiple myeloma cell growth, survival, drug resistance and migration in the bone marrow milieu The cell effect is due to direct multiple myeloma and bone marrow stromal cell interaction , as well as induction of cytokines Cytokines involved include IL6, insulin like growth factor 1, vascular endothelial growth factor stromal cell derived growth factor Growth, drug resistance and migration are mediated via Ras/Raf/mitogen- activated protein kinase, PI3-K/Akt and protein kinase c signaling cascades The clinical manifestation of all the plasma cell disorders relate to expansion of the neoplastic cells secretion of cell productsimmunoglobulins, lymphokines Host’s response to the tumour Bone pain most common complaintprecipitated my movement Weakness Weight loss Anaemia and thrombocytopenia Peripheral neuropathy Hypercalcaemia Renal failure Pathological fractures Symptoms are usually of short duration because of the aggressive nature of the disease The spine is the most common location followed by the ribs and pelvis. Hypercalcaemia, osteoporosis, pathological fracture, lytic bone lesions, bone pain Tumor expansion, osteoclast activating factor, osteoblast inhibitory factors Renal failureHypercalcaemia, light chain deposition, urate nephropathy, drugs Easy fatigue- anaemia Bone marrow infiltration, haemolysis, decreased erythropoietin levels Recurrent infections Hypogammaglobulinaemia, low cd4 count, decreased neutrophil migration Neurologic symptoms Hyper viscosity, croglobulinemia, Hypercalcaemia, nerve compression, POEMS syndrome Nausea and vomiting Renal failure, Hypercalcaemia The classic triad of myeloma is i. Marrow plasmacytosis (>10%)- CD138+, monoclonal ii. Lytic bone lesions iii. Serum and/ or urine M component Monoclonal gammopathies of uncertain significance 1% go on to develop myeloma M protein in serum<30g/l Bone marrow clonal plasma cells<10% No evidence of other B cell proliferative disorder Clinical evaluation of patients with myeloma includes a careful physical examination searching for tender bones and masses. Chest and bone radiographs may reveal lytic lesions or diffuse osteopenia Multiple ‘punched out’ sharply demarcated, purely lytic lesions without any surrounding reactive sclerosis The lack of reactive bone formation is shown by the fact that most lesions are negative on bone scan MRI offers a sensitive means to document extent of bone marrow infiltration and cord or root compression in patients with pain syndromes Histologically multiple myeloma appears as sheets of plasma cells These are small round blue cells clock face nuclei abundant cytoplasm perinuclear clearing or halo Amyloid production can be abundant and may be pathognomonic for the disease Serum immunoelectrophoresis shows monoclonal gammopathy A 24 hr urine specimen quantitate protein excretion concentrated aliquot is used for electrophoresis and immunologic typing of any M component The serum M component will be IgG in 53%, IgA in 25%, and IgD in 1%. 20% of patients will have only light chains in serum and urine. Fewer than 1% of patients will have no identifiable M component The heat test for detecting Bence Jones proteins is falsely negative in 50% of patients with light chain myeloma Complete blood count with differential may reveal anaemia ESR is elavated Serum chemistries calcium, urea, nitrogen, creatinine and uric acid levels may be elevated. Solitary plasmacytoma pathological differentials may include chronic osteomyelitis with abundant plasma cells Plasmcytoma has monoclonal light chains whereas in COM they are polyclonal Myeloma cells stain positive for natural killer antigen CD56, whereas reactive cells do not In poorly differentiated cases lymphoma could be a differential Lymphoma cells usually stain positive for CD45 (leukocyte common antigen) and CD20 ( a B cell marker), Stage 1 Hb>10g/dl, serum calcium <3 mmol/l, normal bone xray or solitary lesion, low M component production Stage Stage 2- neither fitting stage 1 or 2 3- one or more of the following: Hb <8.5g/dl Serum calcium >3 mmol/l Advanced lytic bone lesion High M component production 10% of patients will have an indolent course- slow progression These patients only require antitumor therapy when the disease becomes symptomatic anaemia, hypercalcaemia, progressive lytic bone lesions, progressive rise in serum myeloma protein levels or recurrent infections. Primary treatment of multiple myeloma is chemotherapy Symptomatic bone lesions usually respond rapidly to radiation treatment 40 Gy Treatment of impending or actual pathological fractures of the spine, acetabulum, proximal femur or proximal humerus Patients with symptomatic and/ or progressive myeloma require therapeutic intervention 2 sorts of such therapy Systemic therapy to control the progression of myeloma Symptomatic supportive care to prevent serious morbidity from the complications of the disease Standard treatment for newly diagnosed cases depends on whether the patient is a candidate for high dose chemotherapy with autologous stem cell transplant Transplant candidates avoid alkylating agents such as melphalan Glucorticoids, thalidomide vincristine, doxorubicin, Supportive care directed at the anticipated complications Hypercalcaemia-bisphosphonates, glucocorticoids,hydration, natriuresis Prophylactic IV gamma globulin in recurrent serious infections Anaemia- erythropoietin, along with haematinics Short life expectancy in these patients operations aimed to earliest resumption of full activity Tumor debulking Internal fixation augmented with methacrylate Cemented total joint arthroplasty or hemiarthroplasty In most patients local radiation treatment should be instituted approximately 3 weeks after surgery or when the wound appears to be healed. No conclusive evidence on the corelation Aggressive course and worse prognosis in HIV Multiple myeloma can accelerate progression of HIV infection HIV plays a major role in the evolution of malignant plasma cell tumors Patients with solitary plasmacytoma without evidence of systemic involvement have a better prognosis More than half of patients who present with a solitary plasmacytoma eventually go on to develop multiple myeloma. Patients in stage 1A have a median survival of more than 5 years and those in 3B about 15 months The median overall survival is 5-6 years with subsets of patients surviving over 10 years. The major causes of death are progressive myeloma,renal failure, sepsis or therapy related acute leukaemia or myelodysplasia. Nearly a quarter die of myocardial infarction, chronic lung disease, diabetes or stroke. 2008 2009 2010 2011 2012 ALIVE DEAD 43 41 50 43 48 15 20 22 31 24 TOTAL CASES 58 61 72 74 72 ALIVE DEAD 2008 2009 2010 2011 2012 2 2 1 4 1 1 1 2 1 1 TOTAL CASES 3 3 3 5 2