dengue - AMHOP - Pangasinan Chapter
advertisement

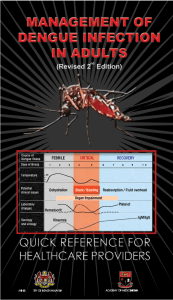
DENGUE: WHO GUIDELINES FOR DIAGNOSIS AND TREATMENT Jimmy Mendigo, MD Infectious Disease Specialist Chrysanta D. Viernes, MD Internal Medicine- ITRMC OBJECTIVES • Discuss the epidemiology and burden of disease of Dengue • Review the transmission • Discuss the new case classification • Discuss algorithms in the diagnosis and management of Dengue EPIDEMIOLOGY • most rapidly spreading mosquito-borne viral disease in the world • incidence: 30-fold increase • increasing geographic expansion to new countries, and from urban to rural settings • 50 million dengue infections annually EPIDEMIOLOGY EPIDEMIOLOGY • 2001-2008: 1, 020,333 cases in Cambodia, Malaysia, Philippines and Vietnam • highest reported deaths in 2008 – Cambodia –Philippines Burden of Disease • WHO estimates that 2.5 billion people— over 40% of the world’s population-- are at risk for dengue infection. • Approximately 50-100 million infections (1 million confirmed) occur each year resulting in 500,000 hospitalizations and 20,000 deaths. Dengue Virus Profile • • • • Genus Flavivirus Family Flaviviridae Single-stranded RNA 4 serotypes (DEN-1 to 4) • 50 nm diameter with multiple copies of 3 structural proteins ( membrane bilayer and single-stranded RNA) Vector Profile • Aedes mosquitoes • A. aegypti • A. albopictus • A. polynesiensis • Tropical and subtropical species • Urban places • Immature stages are found in water-filled habitats The Host • Incubation period: 4-10 days • Primary infection induce lifelong immunity to the infecting serotype • Protection from different serotype within 2-3 months of primary infection • No long-term crossprotective immunity The Host • Individual risk factors: • Age, ethnicity, chronic diseases • Seroepidemiological studies (Cuba and Thailand) • Secondary heterotypic infection • Time interval between infections • Antibody-dependent enhancement of infection Replication and Transmission Blood meal Viral Replication Released in circulation WBC and Lymphatics Replication and Transmission Replication in the salivary gland Viral replication in midgut Female mosquito ingests infected blood Extrinsic Incubation • 8--12 days Dengue Fever • Wide spectrum of clinical presentation, with unpredictable clinical evolution and outcome • Three phases • Febrile phase • Critical phase • Recovery phase • Previously classified into • undifferentiated fever, dengue fever and DHF • Grade 1-IV Dengue Fever Grade 1: fever, non specific constitutional symptoms; (+) TT- only hgic manifestation Grade 2: Grade 1 manifestation + spontaneous bleeding Grade 3: signs of circulatory failure (rapid weak pulse, narrow pulse pressure, hypotension, cold clammy skin) Grade 4: profound shock with undetectable pulse and BP Dengue Fever Febrile Phase Critical Phase Recovery Phase Febrile Phase • Sudden onset of high-grade fever • Lasts for 2-7 days facial flushing skin erythema generalized body ache myalgia and arthralgia headache sorethroat, injected pharynx, and conjunctival injection anorexia, nausea and vomiting Febrile Phase • (+) TT increases the probability of dengue • (+) hemorrhagic earliest abnormality: progressive decrease manifestations in total wbc • enlarged and tender liver Critical Phase • temperature drops to 37.5-38 (days 3-7) • (+) increase in capillary permeability with increasing hematocrit levels • significant plasma leakage lasts for 24-48 hours • progressive leukopenia followed by rapid decrease in platelet precedes plasma leakage Critical Phase • if (-) increase in capillary permeability improve • if (+) increase in capillary permeability pleural effusion and ascites • degree of increase above the baseline hematocrit reflects the severity of plasma leakage Critical Phase • shock: critical volume of plasma is lost • temperature may be subnormal • prolonged shock organ hypoperfusion organ impairment, metabolic acidosis, and DIC severe hemorrhage • severe hepatitis, encephalitis or myocarditis Recovery Phase • gradual reabsorption of extravascular compartment fluid (48-72 hours) • general well-being improves, appetite returns, GI symptoms abate, hemodynamic status stabilizes and diuresis ensues • (+) rash: “isles of white in the sea of red” Recovery Phase • • • hematocrit stabilizes or may be lower due to dilutional effect of reabsorbed fluid wbc starts to rise recovery of platelet count occurs later Approach to the Management • At primary and secondary levels, health care facilities are responsible for emergency/ ambulatory triage assessment and treatment • Triage is the process of rapidly screening patients soon after their arrival in the hospital or health facility in order to identify those – Severe dengue – With warning signs – Non-urgent cases Approach to the Management Approach to the Management • Referral centres receiving severely ill dengue patients must be able to give prompt attention to referred cases. • Beds should be made available to those patients who meet the admission criteria • There should be a designated area to cohort dengue patients, and a high-dependency unit for closer monitoring of those with shock. • Staffed by doctors and nurses Approach to the Management Criteria for transfer: • • • • • • early presentation with shock (on days 2 or 3 of illness); severe plasma leakage and/or shock; undetectable pulse and blood pressure; severe bleeding; fluid overload; organ impairment (such as hepatic damage, cardiomyopathy, encephalopathy, • encephalitis and other unusual complications). Approach to the Management Disease notification • In dengue-endemic countries, cases of suspected, probable and confirmed dengue should be notified • Public health measures – suspected cases • • • • lives in or has travelled to a dengue-endemic area fever for three days or more low ordecreasing white cell counts thrombocytopaenia ± positive tourniquet test. Approach to the Management Management Decisions Groups A • may be sent home • tolerate adequate volumes of oral fluids and pass urine at least once every 6 hours Groups B • referred for in- hospital management • with warning signs, coexisting conditions, • with certain social circumstances Groups C • require emergency treatment and urgent referral • severe dengue (in critical phase) Group A Action Plan • • • Encourage intake of ORS, fruit juice and other fluids Paracetamol and tepid sponge for fever Advise to come back if with no clinical improvement severe abdominal pain persistent vomiting cold and clammy extremities, lethargy or irritability or restlessness, bleeding not passing urine for more than 4–6 hours. monitor: temperature pattern, volume of fluid intake and losses, urine output, warning signs, signs of plasma leakage and bleeding, haematocrit, and white blood cell and platelet counts Group B (with warning signs) Action Plan • reference hematocrit before fluid therapy • isotonic solutions 5–7 ml/kg/hour for 1–2 hours, then reduce to 3–5 ml/kg/hr for 2–4 hours, and then reduce to 2–3 ml/kg/hr or less according to the clinical response reassess: • • haematocrit remains the same or rises only minimally 2–3 ml/kg/hr for another 2–4 hours worsening vital signs and rising haematocrit rising 5–10 ml/kg/hour for 1– 2 hours Group B (with warning signs) Action Plan Give minimum intravenous fluid volume: maintain good perfusion and urine output of about 0.5 ml/kg/hr • • Intravenous fluids are usually needed for only 24–48 hours. Reduce intravenous fluids gradually when the rate of plasma leakage decreases towards the end of the critical phase. monitor: • vital signs and peripheral perfusion (1–4 hourly until the patient is out of the critical phase) • urine output (4–6 hourly) • hematocrit (before and after fluid replacement, then 6–12 hourly) • blood glucose • organ functions (renal profile, liver profile, coagulation profile) Group B (without warning signs) Action Plan • Encourage oral fluids • If not tolerated, start intravenous fluid therapy of 0.9% saline or Ringer’s lactate with or without dextrose at maintenance rate Patients may be able to take oral fluids after a few hours of intravenous fluid therapy. • Give the minimum volume required to maintain good perfusion and urine output. • Intravenous fluids are usually needed only for 24–48 hours. • Close monitoring Group C Action Plan • admit to a hospital with access to intensive care facilities and blood transfusion • plasma losses should be replaced immediately and rapidly with isotonic crystalloid solution or, in the case of hypotensive shock, colloid solutions • blood transfusion: with suspected/severe bleeding • judicious intravenous fluid resuscitation: sole intervention required Group C Action Plan Goals of fluid resuscitation: • improving central and peripheral circulation (decreasing tachycardia, improving BP, warm and pink extremities, and capillary refill time <2 seconds) • improving end-organ perfusion – i.e. stable conscious level (more alert or less restless), urine output ≥ 0.5 ml/kg/hour, decreasing metabolic acidosis. Treatment of Hemorrhagic Complications Patients at risk of major bleeding are those who: • prolonged/refractory shock; • hypotensive shock and renal or liver failure and/or severe and persistent metabolic acidosis • given non-steroidal anti-inflammatory agents • pre-existing peptic ulcer disease • anticoagulant therapy • any form of trauma Treatment of Hemorrhagic Complications • Blood transfusion is life-saving and should be given as soon as severe bleeding is suspected or recognized • Do not wait for the haematocrit to drop too low before deciding on blood transfusion • Risk of fluid overload. Treatment of Hemorrhagic Complications •blood transfusion if with bleeding • 5-10 ml/kg of PRBC or 10-20 ml/kg FWB • repeat if with further blood loss or no rise in hematocrit after transfusion • little evidence to support transfusion of platelet concentrate and FFP • massive bleeding not managed by FWB/PRBC • may exacerbate fluid overload Management of Complications • Fluid Overload Causes: – excessive and/or too rapid intravenous fluids; – incorrect use of hypotonic rather than isotonic crystalloid solutions; – inappropriate use of large volumes of intravenous fluids in patients with unrecognized severe bleeding; – inappropriate transfusion of FFP, platelet concentrates and cryoprecipitates; – continuation of IVF after plasma leakage has resolved – co-morbid conditions such as congenital or ischaemic heart disease, chronic lung and renal diseases Management of Complications Clinical Features: – respiratory distress, difficulty in breathing; – rapid breathing; – chest wall in-drawing; – wheezing (rather than crepitations); – large pleural effusions; – tense ascites; Other investigations: • CXR • ECG • ABG Management of Complications • • Oxygen therapy Stop IVF • When to discontinue IVF: – stable blood pressure, pulse and peripheral perfusion; – haematocrit decreases in the presence of a good pulse volume; – afebrile for more than 24–48 days (without the use of antipyretics); – resolving bowel/abdominal symptoms; – improving urine output • If necessary, give oral or intravenous furosemide 0.1–0.5 mg/kg/dose once or twice daily, or continuous infusion of furosemide 0.1 mg/kg/hour. Management of Complications • If the patient has stable haemodynamic status but is still within the critical phase, reduce the intravenous fluid accordingly. Avoid diuretics during the plasma leakage phase • Patients who remain in shock with low or normal haematocrit levels but show signs of fluid overload may have occult haemorrhage. • Careful fresh whole blood transfusion • repeated small boluses of a colloid solution Criteria for Discharge