ACRIN 6687: 18-F-Fluoride PET as a Pharmacodynamic Biomarker
advertisement

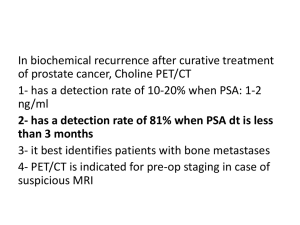
ACRIN 6687 A Phase 2, Multicenter Evaluation of 18F-Fluoride PET as a Pharmacodynamic Biomarker for Dasatinib, a Src Kinase Inhibitor, in Men With Castration-Resistant Prostate Cancer and Bone Metastases Principal Investigators: Evan Y. Yu, MD and David A. Mankoff, MD, PhD Background • ACRIN 6687 is a companion imaging protocol to accompany the therapeutic protocol “Genomic Guided Therapy with Dasatinib or Nilutamide in Metastatic Castration-Resistant Prostate Cancer.” (Dr. Phillip Febbo) supported through the Department of Defense and Bristol-Meyers Squibb • Eligible patients currently enrolled in Dr. Febbo’s therapeutic trial and receiving dasatinib undergo 18F-fluoride PET imaging. – This includes patients genetically indicated to have tumors with SRC microarray signatures who initial dasatinib-alone treatment, as well as those patients with initial AR microarray signatures who may crossover at the time of progression to have dasatinib added to nilutamide. AR Transcriptional Signature Gene expression “Signature” to detect AR activity Febbo et al, Urology (2005) Probability of AR Activity R1881 Metagene Score Mendiratta et al, JCO (2009) AR Transcriptional Signature Neoadjuvant No Treatment After Castration Metastatic CRPC No Treatment Castration Resistant AR activity correponded to tissue DHT levels Data from Holzbelerlein et al. Am J Path (2004) and Stanbrough et al Cancer Res (2007). Study Schema 18F-Fluoride 18F-Fluoride PET PET PCCTC and ACRIN Collaboration • Metastatic, Castration Resistant Prostate Cancer • Disease Amenable to Biopsy • Castrate Testosterone Levels B i o p s y A R A c t i v i t y Nilutamide 150 mg PO QD Dasatinib 100 mg PO QD P r o g r e s s i o n 18F-Fluoride 18F-Fluoride PET PET Dasatinib 100 mg PO QD Nilutamide 150 mg PO QD P r o g r e s s i o n Goals • Potential Insight Gained From the Trial: – 18F-fluoride PET evaluation of an agent, such as dasatinib, that is expected to affect both normal bone and bone metastases – Gain pharmacodynamic information with 18Ffluoride PET on the effects of dasatinib both on normal bone and bone metastases 18F-fluoride – Determine whether PET has prognostic and response biomarker potential for patients with bone metastatic prostate cancer Endpoints • Primary Endpoint – Determine if changes in regional fluoride incorporation, measured by 18F-fluoride PET (SUV and Ki), occur in both castration-resistant prostate cancer bone metastases and normal bone as a response to treatment with dasatinib • Secondary Endpoint: – Determine if changes in 18F-fluoride transport (K1), an indicator of blood flow, and therefore, an indirect marker of angiogenesis, occur in both castration-resistant prostate cancer bone metastases and normal bone as a response to treatment with dasatinib ACRIN 6687 Current Status • Accrual to Treatment Trial • Current Accrual: 54 (N= 60) • 6687 Imaging Protocol • Current Accrual: 12 (N=24) • A total of four (4) centers are approved to participate (UW, Duke, DFCI, OHSU) – Most recent site approval 9/24/10 – One site was unable to bring up the trial due to late timing of protocol approval – Potential to accrue at least two (2) of the accrued 54 as they may cross over to add on Dasatanib to ongoing Nilutamide – One (1) patient out of the 12 did not receive both PET images • Challenge: to reach 24 patient accrual goal – current discussions to expand therapeutic study by 10 patients Challenges • Challenges • Setting up IND tracer availability • Companion Imaging on a subset of patients • Delays to Site Readiness • IRB approval for both the treatment and Imaging Trial slow • Two (2) centers had IRB approval of the treatment trial before the imaging trial and both accrued to treatment trial rapidly • Radiology Interest • Barriers to Accrual • Inclusion/Exclusion to original Febbo trial – (protocol was amended) • Unable to accrue beginning patients as treatment trial was opened prior to imaging trial • Prostate cancer patients are elderly and many have painful bone metastases - reluctance to lay in scanner for extended time Plans For Next Year - 2011 • Completion of Accrual – Target by end of 2010 although treatment study may expand • Complete PET Imaging and Data Collection for 6687 Patients • Finalize Data Sharing with the Febbo Trial • Continue Kinetic Analysis of PET Images • Central Review of PET and Bone Scan Images Thank You