Programme
1. Hematological malignancies: acute leukemias,
lymphomas
2. Solid tumors, part I: Brain tumors, neuroblastoma,
Wilms tumor, hepatoblastoma, germ cell tumors
3. Solid tumors, part II: soft tissue sarcomas,
osteosarcoma, Ewing sarcoma, late effects after
anticancer treatment
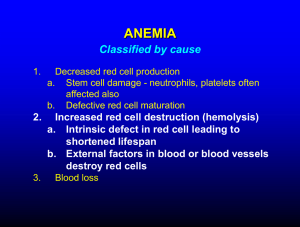
4. Anaemias
5. Coagulation disorders
Pediatric oncology and hematology
Hematological malignancies:
Acute lymphoblastic leukemia
Acute non-lymphoblastic (myeloblastic)
leukemia
Non-Hodgkin lymphoma
Hodgkin lymphoma
Childhood leukemia
Uncontrolled proliferation of immature blood cells
with a different immunological subtypes which is
lethal within 1 –6 months without treatment
The disorder starts in the bone marrow, where
normal blood cells are replaced by leukemic cells
Morphological (FAB), immunological, cytogenetic,
biochemical, and molecular genetic factors
characterize the subtypes with various response to
treatment
Incidence
Most frequent neoplasm in children (28 – 33%)
45/ 1million children under the age of 16 years
Incidence peak at 2 – 5 years
75-80%- acute lymphoblastic leukemia -ALL
15-20% - acute myelogenous (non-lymphoblastic)
leukemia
AML/ ANLL
<5% - undifferentiated acute leukemia and chronic
myelogenous leukemia -CML
Ethiology
Unknown
Higher risk in congenital disorders:
-trisomy 21 (14 times higher) and other trisomies
-Turner syndrome
-Klinefelter syndrome
-monosomy 7
-neurofibromatosis type 1
-Fanconi anemia (high fragility of chromosomes)
-Bloom syndrome, Kostmann S., Shwachman-Diamond S.,
-ataxia- teleangiectasia
-congenital agammaglobulinemia
-Wiskott- Aldrich S.
Ionizing radiation (atomic bomb developed high
incidence of leukemia)
Chemical and drugs:
-benzene
-chloramphenicol
-alkylating agents
Infection (viral –HTLV, EBV, HIV)
Immunodeficiency:
agamma/hypogammaglobulinemia, Wiskott-Aldrich S,
HIV infection
Acute lymphoblastic
leukemia
80% of leukemias
Girl – to- boy ratio is 1: 1.2
Peak incidence 2 – 5 years
Incidence in white children is twice as high as in
nonwhite children
Clinical manifestation
1.
General aspects:
- history and symptoms reflect:
the degree of bone marrow infiltration by
leukemic cells
and
2.
the extramedullary involvement of the
disease
- the duration of symptoms is days to several weeks,
occasionally – several months
- often: low –grade fever, signs of infection, fatigue,
bleeding, pallor
The symptoms depend on the degree of cytopenia:
- anemia: pallor, fatigue, tachycardia, dyspnea,
occasionally- cardiovascular decompensation
- leukopenia:infections, temperature elevation
- thrombocytopenia: petechiae, mucosal bleeding,
epistaxes, prolonged menstrual bleeding
Specific signs and symptoms
Eye: bleeding, infiltration of local vessels,
CNS: at time of diagnosis less than 5%
have CNS leukemia with meningeal signs
(morning headache, vomiting, papilla
edema, focal neurological signs)
Ear, nose, throat:
-lymph nodes infiltration (isolated or
multiple)
-Mikulicz syndrome (infiltration of salivary
glands and/or tear glands)
Laboratory:
Lk 3,91
Er 2,91
Hb 9,2
Ht 25,1
Blood smear: neutr-9% ly- 45% bl-46%
LDH 976
Skin:
maculopapular skin infiltration,
often of deep red color (infants)
Cardiac involvement:
-leukemic infiltration or hemorrhage
-occasionally cardiac tamponade due to
pericardial infiltration
-tachycardia, low blood pressure or other
signs of cardiac insufficiency
Mediastinum:
-enlargement due to leukemic infiltration by
lymph nodes and /or thymus (observed in
T-cell leukemia)
Pleura/and pericardium: effusion
Kidney enlargement
Lymphadenopathy
Gastrointestinal involvement:
-hepato- and/or splenomegaly
Testicular involvement: enlargement of one
or both testes without pain , hard consistency
Penis: priapism is occasionally associated with
elevated WBC
Bone and joint involvement:
-bone pain initially present in 25 % to
50% of patients !
bone or joint pain, sometimes with swelling and
tenderness due to leukemic infiltration of the
periosteum.
Differential diagnosis:
rheumatic fever, rheumatoid arthritis
-radiological changes: diffuse demineralization,
osteolysis,
Laboratory findings
Red cells:
-hemoglobin – normal/ moderate /markedly low
-low number of reticulocytes
White blood cell :
- normal/ low/ high
-in children with high WBC- leukemic blast cells
present
Platelets:
-usually low
Coagulopathy:
-in children with hyperleukocytosis
-more common in AML
-low levels of prothrombin, fibrinogen, factors V, IX,
and X may be present
Chemistry:
-the serum uric acid is often high initially
- the serum potassium level may be high (cell lysis)
-serum hypocalcemia or hypercalcemia (in marked
leukemic bone infiltration)
abnormal liver function > increased level of
transaminases
Bone marrow analysis: >25% blasts
-characterize the blast cells
-determine the degree of reduction of normal
hematopoiesis
-morphological, immunological, biochemical, and
cytogenetic analyses
Differential diagnosis: aplastic anemia,
myelodysplastic syndrome, neoplastic infiltrations
(neuroblastoma, NHL)
Leukemic cell characterization and
classification:
Morphology: FAB classification:
ALL - L1,
ALL-L2,
ALL-L3
AML M0 – M7
chemistry:
ALL:
+ periodic acid Schiff(PAS)
AML:
+ Sudan black, + peroxidase
Immunological characterization:
-monoclonal antibodies to leukemia-associated
antigens differentiate between types of leukemic
cells:
* lympoid stem cells: CD19, HLA-DR, CD 24 (+/-)
* early pre-B cells: CD19, HLA-DR, CD24
* pre-B cells: CD19, HLA-DR, CD24, CD10,
CD20(+/-)
* B-precursors cell: CD19, HLA-DR, CD24, CD10,
CD20
* T-cell lineage: CD7, CD2, CD1, CD4, CD8,CD3
Cytogenetic characterization:
- in 85% of children abnormal karyotype in the
malignant clone
*t(9;22) (BCR-ABL) –unfavorable prognosis
*t (4;11) in infants , poor prognosis
-ploidy and structure of chromosomes
(rearrangements) -hypoploidy- poor prognosis
-DNA index (DI)
Prognostic factors
Favorable:
WBC <10x10 9/l
Age 2-7
Female
Response on steroid (+)
Pre-B-ALL
Hyperploid
FAB L1
↑LDH moderate
Unfavorable:
WBC >50 x 10 9/L
Age < 2 and >10
Male
Response on treatment (-)
Hypoploid, t(9;22)/t(9;11)
FAB L2/L3
↑↑LDH high
visceromegaly
Differential diagnosis
Leukemic reaction in bacterial infection, acute
hemolysis,tuberculosis, sarcoidosis, histoplasmosis
Lymphocytosis: pertussis
Infectious mononucleosis
Aplastic anemia
Idiopathic thrombocytopenia
Bone marrow infiltration by a solid tumor (NBL,NHL,
RMS)
Rheumatoid arthritis, rheumatoid fever
Therapy
In experienced center
Subdivided into:
-remission induction
-consolidation with CNS prophylaxis
-maintenance phase
Prognosis
Rate of first remission in ALL: more than 90%
80% of children survive without relapse
Response on treatment
Reaction on steroids (7.day)
Reaction on chemotherapy (15. and 33. day)
Minimal residual disease - MRD (-)
Acute myelogenous
leukemia
Heterogeneous group of malignant hematological
precursor cells of the myeloid, monocytic, erythroid
or megakaryocytic cell lineage
Epidemiology: 15-20% of all leukemias in children
Frequency remains stable throughout childhood
with slight increase during adolescence
No difference in incidence between boys and girls
FAB classification
M0:
M1:
M2:
M3:
M4:
M5:
M6:
M7:
immature myeloblastic leukemia
myeloblastic leukemia
myeloblastic leukemia with signs of maturation
promyelocytic leukemia
myelomonocytic leukemia
monocytic leukemia
erythroleukemia
megakaryocytic leukemia
Cytogenetics
FAB
Chromosomal
abnormalities
Affected
gene
Comments
M1/M2
t(8;21)
ETO-AML 1
Auer rods
M3
t(15;17)
t(11;17)
PML-RARA
Promyelocytic
leukemia
M4or M5
t(9;11)
AF9-MLL
Infants, high
initial WBC
M5
t(11q23)
MLL
Infants, high
initial WBC
M5
t(1b;11)
AF10-MLL
Infants, high
initial WBC
M5
t(11;17)
AF17-MLL
Infants, high
initial WBC
M7
t(1;22)
Infants with
Down syndrome
Prognostic factors
Favorable
Unfavorable
WBC
<100,000
>100,000
FAB class
M1, M3, M4 with
eosinophils
Infants with 11q23,
Secondary AML, CNS
involvement
Chromosomal
abnormalities
t(8;21) and
t(15;17),inv(16),
t(9;11)
Wild-type FLT3
Mutation of FLT3
receptor,
t(9;22),
del(7)and del(11)
Ethnicity
White ethnicity
MRD (-)
Rapid response to
therapy
Black ethnicity
MRD(+)
Clinical presentation
Bleeding: thrombocytopenia + coagulopathy (DIC)
Leukostasis in the lungs or CNS
Tumor lysis syndrome
Granulocytic sarcoma (chloroma)
Infection (fungal, opportunistic)
Therapy
Induction/ consolidation/ intensification/ maintenance
- in AML3 + ATRA
Allogeneic/ autologous stem cell transplantation
Prognosis
5+year survival rate 50-60 %
Non-Hodgkin lymphoma
(NHL)
Neoplasia of the lymphatic system and its
precursor cells with genetically disturbed regulation,
differentiation and apoptosis
If marked bone marrow involvement is present the
clinical condition is equal of leukemia
Incidence 5 –7 % of all neoplasias in childhood
Peak incidence between 5 and 15 years
Ratio of boys to girls 2:1
Burkitt lymphoma (BL): endemic form in Africa
10:100,000 children and sporadic form in Europe
and USA
Etiology, pathogenesis and
molecular genetics
Often chromosomal alterations are detecable: in B-cell NHL
translocation of chromosome 14 - t(18;14)
Predisposing factors for NHL:
Acquired immunodeficiency: autoimmune disorders, HIV
infection
EBV infection
Congenital B-cell defect, congenital T-cell defect with thymus
hyperplasia
Bloom syndrome, Chedak-Higashi syndrome, SCID, ataxia
teleangiectasia, Wiskott-Aldrich syndrome
Exposure to irradiation
Drug induced, after immunosuppressive treatment
WHO classification
Histology
Rate Immuno phenotype
Main occurence
Burkitt lymphoma
Burkitt-like
lymphoma
50%
Abdomen
Large B-cell
lymphoma
7-8% B-cell
Lymphoblastic
lymphoma
30%
B-cell
Pre-T-cell or
pre-B-cell
Anaplastic, large cell 7-8% T-cell
lymphoma
Thorax, lymph
nodes, bone
Lymph nodes, skin,
soft tissue, bone
Burkitt lymphoma
Burkit-like lymphoma
About 50% of NHL
Localization: abdomen, lymphatic tissue of adenoids and tonsils
80% with translocation t(8;14) or t(8;2) and t(22;8) with c-MYC
on chromosome 8q24 which stimulates proliferation
40% with a p53 mutation
Large B-cell lymphoma
7-8% of NHL
Localization: abdomen, peripheral lymph nodes, skin, bone
Lymphoblastic lymphoma
30% of NHL
Usually mediastinal localization
Anaplastic Large Cell Lymphoma
7-8% of NHL
Clinical manifestations
Duration of symptoms: usually a few days to weeks
Non-specific symptoms: fatigue, nausea, anorexia, loss of weigth
and/or fever
In relation to localisation of NHL:
Abdomen:
especially the ileocecal region, mesentery, retroperitoneum,
ovaries > painfull, spasms, vomiting
Obstipation, intussusception
Apendicitis-like
Ileus, ascites
Mediastinum:
Mostly anterior or middle part of mediastinum > cough, stridor,
dyspnea, wheezing
Edema of the neck and face with marked dyspnea may indicate
SVCS
Pain of the back or abdomen
Pleural effusion
Involvement of adenoid and tonsils, nasopharyngeal
lymph nodes, parotid gland swelling
Peripheral lymph nodes:
Mostly cervical, supraclavicular and inguinal
Lymph nodes are firm, not usually tender, but involving
multiple lymph nodes that usually occur unilaterally
Other locations:
CNS, cranial and peripheral nerves, skin, muscles,
bone, thorax, gonads, parotid gland, epidural region→ spinal
cord compression
Differential diagnosis
Lymph node enlargement in infectious diseases
Autoimmune lymphoproliferative syndrome
Hodgkin Lymphoma
metastatic disease of sarcomas or neuroblastomas
ALL: if more than 25% blasts = ALL, if less= NHL IV stage
Diagnosis:
-histology
-stage
Histological (lymph nodes, peripheral blood, bone marrow or
fluid resulting from pleural effusion or ascites)
In abdominal stage: laparotomy
In SVCS- emergency situation, noninvasive biopsy or
pretreatment with chemotherapy or/and radiotherapy
Morphological, immunophenotypical and molecular /cytogenetic
analyses
Serum lactate dehydrogenase (LDH)
Serum uric acid
Bone marrow aspiration
CSF analysis
Radiological diagnosis
Ultrasound
Conventional X-ray
CT of the thoracic, abdomen and skeletal disease
MRI for CNS
PET (positron –emmision tomography)
Bone scan
Staging ( Murphy/St.Jude)
I- a single tumor (extranodal) or single anatomical area (nodal),
excluding mediastinum or abdomen
II- a single tumor (extranodal) with regional involvement
On same side of diaphragm
a/ two or more nodal areas
b/ two single (extranodal) tumors with or without regional node
involvement
A primary gastrointestinal tract tumor (usually ileocecal) with or
without associated mesenteric node involvement; gross
complete resection
III- On both sides of the diaphragm:
a/two single tumors (extranodal)
b/two or more nodal areas
ALL primary intrathoracic tumors (mediastinal, pleural, thymic)
All extensive primary intra-abdominal disease, unresectable
All primary paraspinal or epidural tumors regardless of other
sites
IV- Any of the above with initial CNS or bone marrow
involvement (less than 25%)
Stages I + II: 10 – 20% of all NHL
Stages III+ IV: 80 – 90% of all NHL
Treatment
Induction therapy should be begun as soon as possible!
Tumor lysis syndrome prophylaxis or treatment
Chemotherapy (in Poland -according to BFM protocols)
Surgical procedure: total resection in I or II stage with localized
masses only
BMT ( auto)
Overall long-term survival >80%
Hodgkin Disease
Progressive, painless enlargement of lymph nodes
with continuous extension between lymph node
region
Pathogmonic histologically :Reed-Sternberg cells
Incidence: 5-7% of all neoplasia in childhood
Boys more than girls
Rare before 5 years; increasing until the age of 11
years
Peak incidence between 15 and 35 years of age
Etiology and pathogenesis
Correlation with EBV infection, genetic predisposition, disturbed
humoral and cellular immune response
High incidence in patients with LE, rheumatoid disorders,
ataxia teleangiectasia, agammaglobulinemia
Clinical presentation
Painless enlargement of lymph nodes, mostly in the cervical and
supraclavicular regions
Swollen lymph nodes are firm, not inflammatory and painfull to
palpation
Most common involved lymph nodes: cervical (75%),
supraclavicular(25%), axillary, infradiaphragmatic
Extranodal involvement: lung, bone, liver
In mediastinal involvement: a cough, sometimes with dyspnea,
dysphagia and enlargement of the vessels of the neck (SVCS)
B symptoms (in 20 -30%)
Fever higher than 38° C
Night sweats
Loss of more than 10% body weight
Sometimes: pruritus and/or nausea
Open biopsy Not fine-nedle biopsy!!!
Histological classification:
Lymphocyte predominance
Nodular sclerosing
Lymphocyte-depleted
Mixed cellular
Laboratory analyses
Blood
Bone marrow
Ferritin, LDH
Immunological analyses
Stage
Radiological evaluation
Chest (x-ray, CT)
Abdomen (usg, CT)
Bone scintigraphy
PET-CT
HL
Staging classification
I: involvement of a single lymph node region(I) or a single
extralymphatic organ (IE)
II:two or more lymph node regions on the same side of the
diaphragm (II) or localized involvement of an extralymphatic
organ or site one or more lymph node regions on the same side
of the diaphragm
III: involvement of lymph node regions on both sides of the
diaphragm (III) which may be accompanied by involvement of
an extralymphatic organ (IIIe) or site, or both (IIIES)
IV: diffuse or disseminated process
A: absence of B symptoms
B: presence of:loss of 10% or more body weight in 6 months
preceding diagnosis, unexplained fever, drenching night-sweat,
pruritus
Differential diagnosis
Toxoplasmosis, tuberculosis, atypical infections
NHL
Mononucleosis
Metastatic disease
Thymus hyperplasia
Rheumatoid arthritis, LE
Sarcoidosis
Treatment
Procedure depends on stage and histopathology
Chemo- and radiotherapy (EuroNet protocol)
Prognosis
Stage I/II EFS >90%
Stage III/IV EFS 70-80%