06-Visconti
advertisement

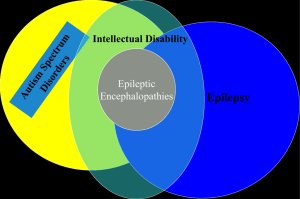
41° Congrès SENP 18-20 avril 2013 Manifestations de type autistique révélant ou associées une maladie neurologique organique P. Visconti UOC NPI IRCCS Istituto delle Scienze Neurologiche Bologna Autism first described by psychiatrist Leo Kanner in 1943 in US Hans Asperger also described “autistic psychopathology” in 1944 in Austria (1980 translated to recognize ‘Asperger syndrome’) Autism was once seen as a rare condition Form of schizophrenia Attributed to poor mothering- Bettleheim, 1960’s Today - Autism Spectrum Disorders (ASDs) having a biologic basis and broad spectrum Autism Spectrum Disorder or Autistic Continuum • Wing e Gould (1979): triad a) Impairments in social interaction b) Impairments in verbal and nonverbal communication, especially regarded to communicational intent c) Poor and stereotyped immagination. • Several levels of mental retardation • “Continuum” of clinical patterns “Autism Spectrum Disorder” (ASD) (Wing, L., 1988) • DSM-V Proposed changes 1. from Pervasive Developmental Disorder (introduced in DSM III-R, APA 1987) to Autism Spectrum Disorder 2. Creation of a single diagnosis, Autism Spectrum Disorder 3. Rett Disorder is eliminated due to the identification of its molecular basis DSM-V Proposed changes Lord et al, 2012 Is there an autistic epidemic? The recent upward trend in estimates of prevalence cannot be directly attributed to an increase in the incidence of the disorder. Confounding factors: •changes in diagnostic criteria; •diagnostic substitution; •increased efficiency over time in case identification; •changes of age at diagnosis; •changes in the policies for special education; •the increased availability of service. Epidemiology … • 1970: 2-5/10000 • All PDDs: 60-70/10000 (=1/150) Fombonne, 2009 • AD: 20-60/10000 (4-30 times) High incidence (> dyabetes, > tumors, > HIV overall) Autism Prevalence (per 1,000) Comparison of Autism Prevalence 01 02 0 3 18 16 Kanner 14 Rutter 12 DSM-III 10 DSM-IIIR 8 ICD-10 6 DSM-IV 4 2 0 1960 1970 1980 1990 Year 2000 2010 2020 Points to remember No biological test to confirm diagnosis at present Diagnosis based on developmental history and observable behaviors and a specific neurobehavioral assessment (Test and scales) Presentation of autism changes with development Which are the relationships among: • Autistic features / Autistic clinical pattern • Syndromic and Non Syndromic Autism • Comorbidities • Underlying Neurological Diseases ??? Comorbidity Low-functioning (QI 70 cut-off) Intellectual Disability High-functioning Mood Disorders Learning disabilities Hyperactivity/ADHD Anxiety Disorder Gastrointestinal Disorders Oppositional Defiant Disorder Sleeping Disorder Stereotyped Movement Disorder Tics Epilepsy OCD Rare Pathologies (Genetic and Methabolic Disorder) S. Tourette Gillberg, 2010 Gillberg, 2010 Gillberg, 2010 Double Syndromes – ASD Center Sample 2000-2005 (255 pts) N. Genetic Syndromes (19) 6 Down 2 Inv-Dup 15 2 Rett-LiKe 2 FG 2 X-Fragile 1 Sotos 1 22 chr. Microduplication 1 Becker 1 Tuberous Sclerosis 1 Rubinstein-Taybi Double Syndromes – ASD Center Sample 2000-2005 N. Infections (2) 1 Rubella 1 Herpes simplex Mixed comorbidity (9) 1 Celiac Disease 1 Congenital Megacolon 1 Blindness 1 Neurosensorial Deafness 3 Epileptic Encefalopathies 1 OCD 1 Tourette Double Syndromes – ASD Center Sample (2000-2005) Double Syndromes Sample: 255 pts. Tot. 30 11,7 % low-functioning Brain MRI abnormalities – ASD Center Sample 2000-2005 255 pts. N. Type (40) 7 Cerebell. Vermis Hypoplasia (rare Cerebell. Hemispheres) 13 Periventricular White Matter Hyperintensity 5 Arnold-Chiari 1 3 Size Ventricular Asimmetry 4 Dysplasias (Hyppocampus and Parietal Region) 3 Delay or not completed Myelination 5 Other Brain MRI abnormalities ASD Center Sample 2000-2005 Abnormalities Sample: 255 pts. Tot. 40 15,7% low-functioning Diagnostic Assessment Neurological Evaluation Protocol • Objective neurological examination • Family Anamnesis • Steps in psychomotor development Anamnesis • Remote and recent Pathological Anamnesis Hematic Laboratory Examinations: • Routine screening (blood count, glycemia etc..) • electrolytes • ceruloplasmin, ammonium, uric acid, lactic acid, pyruvic acid • CPK, LDH • immunoglobulins AGA, EMA, antitransglutaminase • TSH, FT3, FT4 Neurological Evaluation Protocol Urinary Laboratory Examination: uricosuria electrolytes O. R. L. Examination: es. audiometric, otoemissioni, es. impedenziometrico Ophthalmic Evaluation Neurogenetic Screening: Serum and urinary amino acids Lysosomial leukocytary enzymes Oligosaccharides, Mucopolisaccharides and glycosaminoglycans Neurophysiological Examination: EEG (awake/aspleep) ABR and other evoked potentials, if indicated Neurological Evaluation Protocol Genetics: - Clinical evaluation - X Fragile (Fra-X A and E) If indicated, high resolution karyotype MECP2 Focused investigation on specific pathologies (S. di Angelman) Array-CGH Radiological examination and diagnosis thorough Images: - Rx for the bone age evaluation - Cerebral MRI Epilepsy and autistic spectrum disorders (ASD) often occur together The prevalence of epilepsy in all children is 2-3% Prevalence of epilepsy in autism from 5 to 42% (Rosman and Bergia, 2013) Frequency in HF ASD lowest at 11% (Tuchman and Rapin, 1997) Frequency in LF ASD is highest at 39% (Kawasaki et al.1997) Epilepsy Autism • Mouridsen SE et al, 2011: epilepsy prevalence was greater in association with severity of ID 34% of epilepsy in Pts with autism and IQ< 50 27% of epilepsy in Pts with autism and IQ 50-69 9% of epilepsy in Pts with autism and IQ> 70 Autism Epilepsy Several small series report the occurrence of autism or autistic features in patient with selected types of epilepsy: •Infantile spasms •Tuberous sclerosis with mutation in TSC2 gene •Dravet syndrome •Epilepsy in female with mental retardation in PCDH19 Intellectual disability is the main and possibly the only reason that studies find a higher-than-expected level of autism in children with epilepsy Absent ID, the overlap is no greater than expected by chance alone . The question is: Above and Beyond any contribution from ID Have ASD and Epilepsy a special relationship ? ? • Might there be common underlying pathophysiological mechanisms that can explain the frequent cooccurrence of these two conditions? ASD and epilepsy disorders of synaptic plasticity that result in imbalances of excitation and inhibition in the developing brain •Fragile X •Rett syndrome •CDKL5 mutations • tuberous sclerosis complex (TSC) • neuroligin mutations •“interneuronopathies” resulting from anstalessrelated homeobox, Xlinked (ARX) and Neuropilin 2 (NRP2) gene mutations. Regression Regression is noted usually at 18-24 months with particular loss of verbal and nonverbal communication. EEG abnormalities occur in 20-40% with autism and regression and in 6-30% of those with autism without regression (Kagan-Kushnir T, 2005) Rates of epileptiform discharges are influenced by the types of EEGs performed with highest rates in studies using overnight recordings or include at least some sleep state recording Autism and Metabolic Diseases (1) Inherited metabolic disease (IMD) with isolated ASD as a prominent feature or as a first sign (Schiff et Al, 2011) • • PKU + behavioural disorder (BD), epilepsy, severe ID HCY (Homocystinuria) + lens subluxation, vascular thrombosis, skeletal abnormalities • treatable treatable Mucopolysaccharidosis type III (MPS III, San Filippo Disease) + severe BD, slowly progressing developmental impairment, speech regression, loss of toilet training → severe encephalopathy • Urea Cicle Disorder (Ornithine transcarbamylase deficiency) + BD, ataxic gait, epatho-digestive abnormalities • treatable Cerebral Creatin Deficiency Syndrome (CCTD) + ID, Oral Dyspraxia, speech delay Autism and Metabolic Diseases (2) • Adenylosuccinase Deficiency (autosomal recessive error of purine synthesis) + developmental delay, seizures, agitation (Vincent e Jackson, 1997; Sempere et Al, 2010) • X-linked Adreno-leukodystrophy (Schilder Disease) (Rosman and Bergia, 2013) • Metachromatic leukodystrophy (Rosman and Bergia, 2013) CCTD screening 100 Autistic Pts (Newmeyer et al., 2007) No pathogenetic (“silent”) mutation 290 pts with X-linked ID Prevalence: 2.1% (6/288) 2004) (Rosenberg et al., • • • • • Is an Autistic phenotype ? 9 y.boy with no medical or psychiatric history Acute onset of secondary generalized seizure Speech and swallowing difficulties 10 days later agitated catatonic state with opisthotonic posturing, tonic posturing of limbs, insomnia, dyskinesia. + Oligoclonal bands in CSF, treatment for Atypical child. Epilepsy (CT spikes) • 1 month later: Robotic state, complete mutism and negativism Final «organic» diagnosis • Marked deterioration, catatonia, a normal development up to at least 5 years of age suggest an ORGANIC CAUSE • Slightly raised anti-NMDA-receptor Ab in serum and highly raised in CSF Conclusion Taking in account to investigate metabolic disorders and/or syndromic Autism Spectrum Disorder in presence of these clinical signs: Seizures Dysmorphic features Skin abnormalities Vomiting Ataxia Micro/macrocephaly Cataract Developmental delay and/or ID Children with Autistic Spectrum Disorder who have lower cognitive function are more likely to have specific neurological disorder or epilepsy “Autistic Phenotype” or Comunication-Social Impairment in Moderate/Severe ID ? • Learning disabilities • Absent understanding of environmental requests • Attentional deficits • Degree of delay Autistic features both in low and high functioning ADS pts are caused by an early insult on developing brain BUT In low functioning ADS pts the pathological impact causing the disruption of neurodevelopment trajectories is global and wide, affecting different cortical developing networks In high functioning ADS pts it is more specific on networks subserving social/communication functions What’s the role of the genetic underlying condition? Could autistic phenotype in low functioning pts depend on or be linked to the ID, and not to a specific condition? Is Autism phenotype specific “per se” or is the common final pathway of different pathogenetic mechanisms? Reduced frontal-posterior cortical connectivity. Common finding (Kana et al., 2009); It represents a constraint on the capacity of cortical networks to coordinate information processing (Kana et al., 2006) Disturbances in integrative information processing as the basis for the clinical deficits that define autism. A specific and detailed neurobehavioral and neurological assessment is needed: • • • CARS, ADI, ADOS Core autistic features Neurobehavioral and dimensional profile (language, IQ, executive function, visual-motor integration, adaptive and playing behavior…) Homogeneous ASD subgroups to drive genetic research Un Saluto da Bologna!