Cell of Origin subclassification for DLBCL – if we can
advertisement

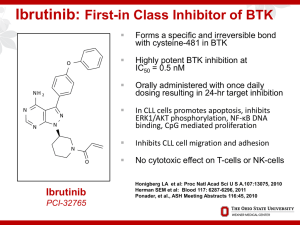
Diffuse Large Cell Lymphoma Cell of Origin – Ready for Prime Time? Thomas Witzig, MD Hematology Malignancy Program Mayo Clinic Cancer Center Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida Disclosure 2 Research funding from Celgene, Novartis, Millenium for clinical trials Research funding from Millenium for preclinical work Advisory boards for Spectrum, Celgene, and Bayer (no personal compensation) Origins Research in DLBCL Time for Action? Lancet Oncol. 2014 Jun;15(7):674-5 Cell of Origin Techniques Gene expression profiling Microarrays on RNA expression Frozen tissue Thousands of genes Year 2000 onward Immunohistochemistry 2004; Many algorithms; Hans still most widely used 2014 - Lymph2Cx Blood. 2014 Feb 20;123(8):1214-7 15 Years Ago Nature. 2000 Feb 3;403(6769):503-11. “And like most overnight successes, it was about twenty years in the making” S. Walton originator of WalMart Nature. 2000 Feb 3;403(6769):503-11. Prognostic in the Pre-RCHOP Era Nature. 2000 Feb 3;403(6769):503-11. Hans Method – 10 Years Ago Hans et al Blood. 2004;103(1):275-82. OS by TMA Hans et al Blood. 2004;103(1):275-82. • 262 cases of DLBCL; 192 had GEP Meyer PN et al J Clin Oncol 2011;29(2):200-7. 2011 by American Society of Clinical Oncology Meyer P N et al. JCO 2011;29:200-207 Event-free survival of patients with diffuse large B-cell lymphoma according to immunophenotype by each algorithm. Hans Tally Meyer P N et al. JCO 2011;29:200-207 2011 by American Society of Clinical Oncology Overall survival of patients with diffuse large B-cell lymphoma according to immunophenotype by each algorithm. Hans Tally Meyer P N et al. JCO 2011;29:200-207 ©2011 by American Society of Clinical Oncology Blood. 2014 Feb 20;123(8):1214-7 Lymph2Cx Blood. 2014 Feb 20;123(8):1214-7 Lymph2Cx Matched FFPE and frozen Traditional GEP; IHC; and Lymph2Cx Training cohort – 51 cases Validation cohort – 68 cases 28 GCB; 30 ABC; 10 unclassified 10 micron scrolls; Qiagen AllPrep FFPET kit 20 GCB; 19 ABC; 12 unclassified Extract RNA from FFPE tissue slice Digital GEP on 200 ng RNA using Nanostring technology Sample split and run independently in 2 labs Blood. 2014 Feb 20;123(8):1214-7 Lymph2Cx Tested 93 genes found by Lenz et al to differentiate GCB from ABC (Lenz NEJM 2008) 20 were all that were needed 15 of the 93 and 5 “housekeeping genes” NanoString technology on 20 genes was used in these two datasets Blood. 2014 Feb 20;123(8):1214-7 PFS OS Lymph2Cx Gold Standard GEP Blood. 2014 Feb 20;123(8):1214-7 Cell of Origin Prognosis or Helping Choose Therapy? Which drug to add????? Bendamustine 65 Years of Lymphoma Rx Lenalidomide Everolimus Vorinostat Rituximab RIT Autologous SCT Cis-platinum Methotrexate Pralatrex Romadep CHOP ABVD CHOP Wins! Vincristine Doxorubicin Nitrogen Mustard 1949 RCHOP 2-CDA Bort VP-16 1953 1963 1975 1978 1983 1993 1997 Era of Chemotherapy Brentux 2011 Ibrutinib Lenalidomide 2013 1999 ‘02 03 ‘05‘07 ‘09 Era of Targeted Therapy Idelalisib 2014 R-CHOP is 15% Better than CHOP 1.0 Probability 0.8 R-CHOP 0.6 0.4 HR=0.64 p=0.003 0.2 0.0 0.0 0.5 1.0 1.5 CHOP 2.0 2.5 3.0 3.5 4.0 4.5 Failure-Free Survival 5.0 1.0 R-CHOP Probability 0.8 0.6 CHOP 0.4 Overall Survival HR=0.72 p=0.05 0.2 0.0 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 Years from Induction Randomization Coiffier et al N Engl J Med. 2002; Habermann et al J Clin Oncol 2006 CP1171726-10 R(X)CHOP Era – what is X? 22 Epratuzumab - ERCHOP Lenalidomide – R2CHOP Bortezomib – Bor-RCHOP Everolimus – ER-CHOP; maintenance E in CRADN2301 (enrolled) Everolimus – EverRCHOP – N1085 Ibrutinib – IR-CHOP Ann Oncol. 2014. Epub 2014/03/15 MC078E – Phase II Results Phase I/II trial of R2CHOP for untreated DLBCL Any age RCHOP + lenalidomide 25 mg days 1-10 q21 x 6 cycles ASA daily Pegfilgrastim day 2 No maintenance Cell of origin by Hans algorithm Nowakowski G et al J Clin Oncol. 2014. Epub 2014/08/20 MC078E 64 patients enrolled; 60 evaluable 87 controls at same time with RCHOP ORR 98% (59/60) CR 80% (48/60) Event-free survival at 24 months - 59% Overall survival at 24 months - 78% No difference in GCB vs non-GCB in R2CHOP arm Nowakowski G et al J Clin Oncol. 2014. Epub 2014/08/20 MC078E RCHOP control EFS/OS at two years for GCB: 46% and 78% EFS/OS at two years for non-GCB: 28% and 64% R2CHOP EFS/OS at two years for GCB: 60% and 83% EFS/OS at two years for non-GCB: 59% and 75% Nowakowski G et al J Clin Oncol. 2014. Epub 2014/08/20 R2CHOP R2CHOP RCHOP RCHOP Nowakowski G et al J Clin Oncol. 2014. Epub 2014/08/20 RCHOP RCHOP R2CHOP RCHOP R2CHOP Nowakowski G et al J Clin Oncol. 2014. Epub 2014/08/20 Italian R2CHOP 29 DLBCL and FL 3b R2CHOP in 13 centers in Italy GCB vs non-GCB by IHC (Hans) Standard RCHOP x 6 Lenalidomide 15 mg days 1-14 q 21 49 patients 92% (45/49) ORR with 86% functional CR Lancet Oncol. 2014;15(7):730-7. Outcome of R2CHOP (Italian) 30 Vitolo et al Lancet Oncol. 2014;15(7):730-7. GCB vs. non-GCB 31 Lancet Oncol. 2014;15(7):730-7. ©2011 MFMER | slide-32 ECOG 1412 • Randomized phase II of RCHOP vs. R2CHOP • First patient in September 19, 2013 • GCB and ABC • Endpoint is response in ABC as defined by GEP • Nanostring on paraffin-embedded tissue • 110 patients accrued as of October 2014 ©2011 MFMER | slide-33 DLBCL-002 34 FDA registrational, International Phase III RCHOP x 6 vs. R2CHOP x 6 Lenalidomide 15 mg days 1-14 vs. placebo Untreated DLBCL Stage II-IV Ages 18-80 years Requires excisional biopsy ABC by Nanostring GEP on FFPE tissue Promised 5 day turnaround; steroids allowed 600 patients Opening Dec 2014 Furman RR et al Cancer. 2010;116(23):5432-9. L. Staudt Bortezomib R-CHOP 37 20 patients – 16 DLBCL/4 MCL Median age 66 years (range, 29-84) Standard RCHOP-21 Bortezomib - Days 1 and 4 of each cycle 0.7 mg/m2 - 4 patients 1.0 mg/m2 - 9 patients 1.3 mg/m2 - 7 patients No DLT with any dose; grade 3 neuropathy in 1 95% CR Furman RR et al Cancer. 2010;116(23):5432-9. Bortezomib R-CHOP 38 At a median follow-up of 56 months Overall survival at 4 years was 75% Progression-free survival was 58% Randomized phase II of RCHOP vs. RBCHOP in progress in US Furman RR et al Cancer. 2010;116(23):5432-9. Phase III Trials 39 RCHOP vs. Bor-RCHOP in UK Start May 2011 and predicted to end in 2015 Ibrutinib with RCHOP 40 Younes A et al Lancet Oncol. 2014 Aug;15(9):1019-26. IR-CHOP 41 Phase 1/2 DLBCL, MCL, FL June 2012 – May 2013 No ASA, warfarin, heparin allowed RCHOP x 6 + ibrutinib daily No maintenance No prophylactic G-CSF (allowed but not mandated) Younes A et al Lancet Oncol. 2014;15(9):1019-26 IR-CHOP 42 33 patients No MTD for ibrutinib; thus 560 mg/d continuously with standard RCHOP-21 18% febrile neutropenia 18 pts with DLBCL in the phase 2 Younes A et al Lancet Oncol. 2014;15(9):1019-26 IR-CHOP 43 100% ORR in the DLBCL (18/18) 15 CR and 3 PR 4/4 nonGCB – CR 5/7 GCB – CR PK not affected for either ibrutinib or vincristine A randomized phase III is ongoing –placebo controlled (NCT01855750) for non-GCB type Younes A et al Lancet Oncol. 2014;15(9):1019-26 Summary about COO in DLBCL 44 Increases understanding of the biology Helps predict prognosis but not particularly well May guide therapy New treatments with lenalidomide and ibrutinib are focusing on ABC-type New techniques of GEP will enhance the clinical utility and “bring it to your hospital” Prediction – you will use COO to “choose X”