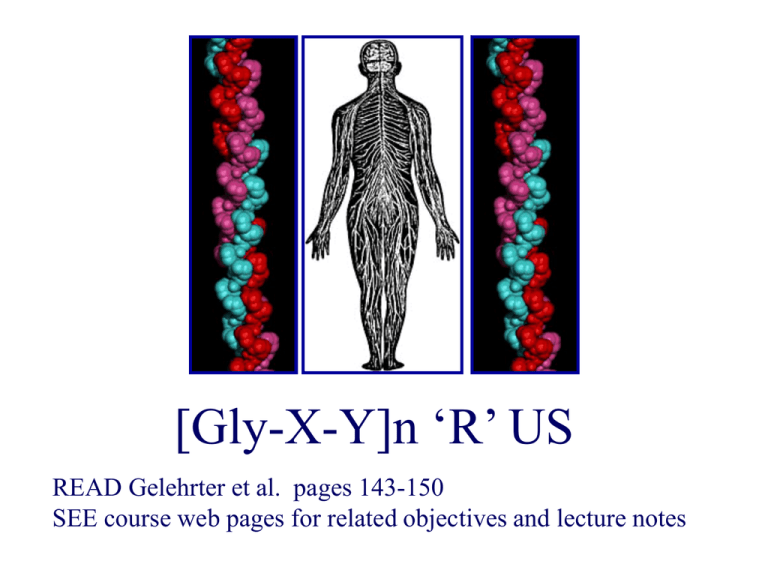
[Gly-X-Y]n ‘R’ US
READ Gelehrter et al. pages 143-150
SEE course web pages for related objectives and lecture notes
Collagen Disorders
• Acquired/Multifactorial
– Aging
• Mendelian Inheritance
– Osteogenesis imperfecta (Type I Collagen)
– Ehlers-Danlos syndromes (Types I, II,III, V Collagens)
– Chondrodysplasias (Type II Collagen)
– Alport syndrome (Type IV Collagen)
– Stickler syndrome (Types II, XI Collagen)
– Schmid metaphyseal dysplasia (Type X Collagen)
– Dystrophic epidermolysis bullosa (Type VII Collagen)
32
Aortic dissection
68
HTN
36
60
55
Vertebral artery rupture 57,
Aortic dissection 60,
33
1
53
4
R internal carotid dissection, cerebral
infarct,
R vertebral artery aneurysm, s/p repair
Abdominal aorta aneurysm/chronic
dissection,
Aneurysm R common iliac
30s
5
Splenic
Artery rupture,
31
5
Arterial rupture
Procollagen
Proelastin
Connective tissue proteins are synthesized in a tissue
specific manner by a variety of specialized cells such as
fibroblasts, chondrocytes, osteoblasts.
What is collagen?
• Major extracellular fibrous protein that provides
strength and structure to tissues by organizing the
extracellular matrix via supramolecular assembly
functions
• A ropelike structure made by intertwining three
polypeptide chains into a triple helix
Fibrillar collagens have awesome tensile strength
Reticular collagens provide a meshwork of support
25 distinct collagen molecules vary by content of
genetically distinct a-chains
•
Type I collagen: [a1(I)]2a2(I)
Heterotrimer
two a-1 chains (COLIA1 on chromosome 17)
one a-2 chain (COLIA2 on chromosome 7)
•
Type III collagen: [a1(III)]3
Homotrimer
two a-1 chains (COL3A1 on chromosome 2)
Connective tissue
supporting
hollow organs
including colon
contains Type III
collagen
Skin contains Type I, III, V
collagens and elastic fibers
Connective tissue surrounding
blood vessels includes type III
collagen and elastic fibers
[Gly-X-Y]n
X: proline, lysine
Y: hydroxyproline, hydroxylysine.
•
•
•
•
Each polypeptide chain is wound in a left-handed helix
Three chains form a triple helix monomer
The helix is stabilized by hydrogen bonds in a right-handed helix.
Importantly, the third residue is glycine, as it can fit into the center of the helix.
disulfide
bonds
The making of collagen…
Polypeptide chains are synthesized from ribosomes, signal peptides are
removed and a series of post-translational modifications take place:
Intracellular modifications:
• Hydroxylation of proline and lysines allows bonding to other molecules which
requires co-factors (i.e. vitamin C).
• Glycosylation of hydroxylysine residues via galactosyl transferase, lysyl
hydroxylase, and glucosyl transferase controls fibrillar morphology.
• The three chains align and disulfide bonds form to form triple helix.
• Triple helix is packaged into a vesicle and secreted from the cell.
Extracellular modifications:
• Calcium dependent N- and C-proteinases remove the propeptides
• Procollagen molecules aggregate by a 1/4 staggering in respect to its neighbor and
are stabilized by covalent cross links.
• A polymeric fibril is formed.
• Fibril is arranged in different tissue specific ways
By EM (50,000 X), collagen appears as
regular striated bundles in longitudinal
section and circles on cross section
Type I collagen fibers
Some collagens that form long structural fibrils
I.
Skin, tendon, bone, dentin; a fetal form also exists
Mutations cause osteogenesis imperfecta, procollagen cleavage mutations
cause EDS
II. Cartilage, vitreous humor
Mutations cause abnormalities of skeleton and eye, Stickler syndrome
III. With type I in skin, also in muscles, blood vessels
Mutations cause Vascular EDS
V. Fetal tissues, placenta, interstitial tissues, skin
Important for extracellular matrix assembly, mutations cause classic C
Classic EDS
Type I collagen
• 90% of our collagen is type I
• two a1(I) chains (COL1A1 chr 17) and one a2(I) chains (COL1A2 chr 7))
• Most abundant in: Bones, skin, tendons, cornea
300nm long
1.5nm in diameter
a1(I)
a2(I)
• COL1A1 and COL1A2 mutations cause Osteogenesis Imperfecta syndromes
• Posttranslational modification defects cause rare Ehlers-Danlos syndromes
Type I collagen fibers
arranged to provide
structural integrity
Type I collagen fibers
Osteogenesis
Imperfecta
• Four major clinical sub-types
(Sillence classification) vary in
phenotypic characteristics and
severity (I,IV,III,II)
• Highly penetrant, inter- and
intra-familial variable
expressivity
• Prognosis varies greatly
depending on type
OI Type I (with or without DI)
• Most common form
• Possible dentinogenesis
imperfecta/opalescent dentin
• Type I collagen is decreased
in amount, generally normal • Conductive hearing loss in up
in structure
to 50%
• Normal/near normal height • Autosomal dominant
• Little or no bone deformity
• Blue/gray sclera
• Increased bruising
• Joint hypermobility
• Possible scoliosis
Osteogenesis
Imperfecta
gray/blue sclera
Opalescent dentin/
Dentinogenesis
imperfecta
OI Type IV
(with or without opalescent dentin)
• Osteoporosis and bone fragility without significantly blue/gray
sclerae after birth or early onset deafness
• 25% have fractures at birth while in some cases fractures do not
occur until later in life
• May be clinically confused with idiopathic juvenile
• Short stature
• Mild to moderate bone deformities
• Scoliosis
• Dentinogenesis imperfecta/opalescent dentin in some patients
• Possible hearing loss, but not as common as in type I
• Autosomal dominant
OI Type III
• Significant fractures at
birth and/or infancy
• Marked short stature
• Severe kyphoscoliosis
with progressive
platyspondyly
• Severe long bone
progressive deformities,
often require wheelchair
• Decreased muscle mass in
arms and legs
• Loose joints
• Whites of the eyes (sclera)
have a blue, purple, or gray
tint
• Brittle teeth
• Possible hearing loss
• Possible cardiopulmonary
problems
• Most autosomal dominant,
• ? Autosomal recessive types
• ? Additional loci
OI Type II
• Most severe form with
extreme bone fragility at
birth
– Continuous beaded ribs
with crumpled long bones
– Crumpled long bones with
few rib fractures
– Narrow, dysplastic beaded
ribs with poorly molded
long bones and fractures
• Frequently lethal at or
shortly after birth
•
•
•
•
•
Numerous fractures
Severe bone deformities
Very small stature
Underdeveloped lungs
Abnormal brain
neuropathology
• Diminished and structurally
abnormal type I collagen
• Most cases autosomal
dominant mutations with
germline mosaicism
Skeletal dysplasia classified according to the region and
type of bone involved
•
Predominantly epiphyseal
–
–
–
•
Predominantly metaphyseal
–
–
–
•
Spondyloepiphyseal dysplasia ‘congenita’
SED (x-linked and others)
Pseudoachondroplasia
Kneist syndrome
Diastrophic dwarfism
Predominant involvement of single sites
–
•
Lethal short limb dwarfism
Achondroplasia
Metaphyseal chondrodysplasias
Major involvement of the spine
–
–
–
–
–
•
Multiple epiphyseal dysplasia
Hereditary arthro-ophthalmopathy (Stickler syndrome)
Chondrodysplasia punctata
e.g. mesomelic dwarfism
Abnormal bone density and/or modelling defects
–
e.g. sclerosing disorders of bone
From: Pope FM, Smith R. Color Atlas of Inherited
Connective Tissue Disorders, Mosby-Wolfe, 1995
OI - Autosomal Dominant
• If a child inherits an
altered collagen allele,
the child will have the
same type of OI as the
parent
= Normal Gene
= altered Gene
Parent with OI
Unaffected Parent
Child with OI
Normal
‘Protein suicide’
typical Type I
typical type II
okay
degraded
How is OI inherited when there is
no family history of OI?
• 5-8% of families without a prior history of OI have
multiple children with OI
• Due to “new” or “spontaneous” alteration occurs in the
gonadal cells which give rise to multiple sperm or eggs.
• Germline or gonadal mosaicism - parent not affected by the
condition, but mutation in certain percentage of his or her
reproductive/germline cells
Clinical Testing
• Diagnostic confirmation of type
• Two available tests for OI
– Protein/Biochemical
– DNA
• Prenatal diagnosis available using ultrasound (OI
type II), amniocentesis or chorionic villus
sampling.
How is OI treated?
• Prevent/control symptoms, maximize mobility, and developing
optimal bone mass and muscle strength.
– Physical therapy, exercise, maintain normal weight
– Proper care of fractures
– Activity guidance - avoid high risk of injury activities
– Pain management
– Surgical placement of metal rods in the bones to provide
strength
– Motility aids, such as wheelchairs and braces
– Avoid smoking, excessive alcohol, steroids
– Regular dental care
• Bisphosphates, calcium, and Vitamin D
Ehlers-Danlos Syndrome(s)
1 in 5,000 - 10,000 people
both males and females
all racial and ethnic backgrounds
• Skin:
soft velvety; hyperextensible; fragile - tears or bruises
easily; severe parchment paper scars; slow and poor
wound healing; molluscoid pseudotumors over
pressure areas, striae
• Joints:
hypermobile/hyperextensible, loose/unstable; prone to
dislocations and/or subluxations; pain; hyperextensible
joints; early osteoarthritis; chronic, early onset,
debilitating musculoskeletal pain, pes planus, scoliosis
• Other:
mitral valve prolapse; blue sclerae, gum disease, organ
and vascular fragility
Six types of Ehlers-Danlos syndrome
• Classic Type (types I and II) AD - Type V and type ? collagen alterations
“Parchment paper” scars, joint hypermobility,skin hyperelasticity, bruising
• Hypermobility Type (type III)
AD - Type ? collagen alterations
Joint hypermobility/extensibility, joint pain, premature arthritis
• Vascular Type (type IV) AD - Type III collagen alterations
Arterial/intestinal/uterine fragility or rupture, bruising, translucent skin
• Kyphoscoliosis Type (type VI) AR - Lysyl hydroxylase deficiency
Scoliosis at birth, scleral fragility
• Arthrochalasia Type (types VII A, VII B) AD - Type I collagen alterations
Hypotonia, congenital hip dislocation
• Dermatosparaxis Type (type VII C) AR - Amino proteinase deficiency
Severe skin fragility, doughy skin, blue sclerae, short stature, severe bruising
Diagnostic Criteria for Classic EDS (I and II)
•
•
•
Autosomal Dominant
Major Criteria
– Skin hyperextensibility
– Widened atrophic scars
– Joint hypermobility
Minor Criteria
–
–
–
–
–
–
–
–
–
•
Smooth, velvety skin
Molluscoid pseudotumors
Subcutaneous spheroids
Joint complications (eg. sprains, dislocations, subluxations, pes planus)
Muscle hypotonia, gross motor delays
Easy bruising
Complications of fragile tissue (hiatal hernia, anal prolapse)
Surgical complications (postoperative hernias)
Positive family history
Laboratory Diagnosis
– Type V collagen abnormalities ( < 50%, most unknown)
– EM with ‘cauliflower’ fibrils
Diagnostic Criteria for Hypermobility Type EDS
•
•
•
•
Autosomal Dominant
Major Criteria
– Generalized joint hypermobility
– Skin hyperextensibility OR smooth, velvety skin (if atrophic scars are
present, probably classic type)
Minor Criteria
– Recurring joint dislocations (frequently shoulder, patella, and TMJ)
– Chronic joint/limb pain - often early onset and possibly debilitating
– Positive family history
Laboratory Diagnosis
– None
• Distinguish from familial hypermobility (?)
Hypermobility EDS
Diagnostic Criteria for Vascular EDS
•
•
•
•
Autosomal Dominant
Major Criteria: Two major criteria - highly indicative of the diagnosis, One - be very
suspicious especially if family history
– Thin, translucent skin
– Arterial/intestinal/uterine fragility or rupture
– Extensive bruising
– Characteristic facial appearance with decreased subcutaneous adipose tissue, particularly in
face and limbs, often with big eyes, pinched nose
Minor Criteria: One or more minor criteria with positive family history, be suspicious
– Acrogeria
– Hypermobility of small joints
– Tendon and muscle rupture
– Talipes equinovarus
– Early onset varicose veins
– Arteriovenous, carotid-cavernous sinus fistula
– Pneumothorax/pneumohemothorax
– Gingival recession
– Positive family history or vascular or organ rupture, sudden early death in relatives
Laboratory Diagnosis: Type III collagen abnormalities in fibroblasts, COL3A1 mutation
Compressed carotid artery by blood
dissecting upward from a dissection.
Blood dissects along the media (asterisks).
*
Translucent skin
31
90s
Ruptured renal artery
“Marfan disease”
35
32
0
43
Vertebral aneurysm
Spontaneous pneumothorax
Sigmoidal perforation
Ruptured thoracic artery
Unaffected
Probable EDS IV
EDS IV- Vascular type
Confirmed by DNA testing
16
15
14
Bilateral inguinal hernias
Easy bruising
Soft, translucent skin
Increased bleeding
17
3
Type III
Collagen
•
•
•
•
Consists of 3 a1(III) chains (COL3A1 on chr 2)
Thin collagen fibers created
Forms a loosely woven meshwork of reticular fibers
Largely in:
skin (with type I and type V collagens)
blood vessels
internal organs (e.g. smooth muscle layers GI tract, uterus, bladder)
Vascular EDS Manifestations (Pepin et al, NEJM, 2000)
• 220 patients, 199 relatives
• Mean age at ascertainment: 28.7
– Patients: 24.9 + 13 (M - 25.1, F - 24.7);
– Relatives: 33.3+15.6
• Family history present:
– Yes: 38.2 %
– No: 41.4%
• Age of first complication in index patients (n=136)
– 23.5+11.1 (M - 23.9, F - 22.8)
– 25% had significant complication before 20 years
– 80% had significant complications before 40 years
• Age at death ranged from 6 -73 years, median = 48
Vascular EDS Manifestations (Pepin et al, NEJM, 2000)
Type of first complication in index patients
Arterial
GI
None
Organ
Vascular EDS Manifestations (Pepin et al, NEJM, 2000)
Causes of death (n =131)
Arterial
Organ
GI tract
Other
Vascular EDS Manifestations (Pepin et al, NEJM, 2000)
Cause of pregnancy related deaths in 12 of 81 women (167 live births)
Maternal death rate 1 in 23 (11.5%)
Arterial rupture at delivery
Uterine rupture during labor
Arterial rupture in postpartum period
Treatment/Management of Vascular EDS
No specific therapies delay onset of complications.
No proven medical surveillance for
complications of Vascular EDS.
The diagnosis should be established to provide genetic
counseling, anticipatory guidance and education, and appropriate
management strategies for surgeries and pregnancies.
Defect in N-Proteinase
Diagnostic Criteria for Dermatosparaxis Type EDS
•
•
•
Autosomal recessive
Major Criteria
– Severe skin fragility
– Sagging redundant skin
Minor Criteria
–
–
–
–
•
Soft, doughy skin texture
Easy bruising
Premature rupture of fetal membranes
Large hernias
Laboratory diagnosis
– Biochemical confirmation of procollagen N-terminal peptidase (N-proteinase) by
analysis of pNa1(I) and pNa2(I) in fibroblasts
– Determine level of N-proteinase activity
Patient 1
Patient 2
Dermatosparaxis
Normal
Genetics of Ehlers-Danlos syndrome
• Classic Type (types I and II)
AD - Type V and type ? collagen alterations
• Vascular Type (type IV)
AD - Type III collagen alterations
• Dermatosparaxis Type (type VII C)
AR - Amino proteinase deficiency
Treatment/Management of EDS
Joint care
Skin care
Medications:
Pain medications (avoid excessive NSAIDS in vascular type)
Ascorbic acid (vitamin C) 1 - 4 g/day in adults, 0.5 to 1.0 mg/day in children
Consider risks/benefits of anticoagulation in vascular type
Medical and Surgical Care:
Be sure all health care providers know about the diagnosis (medic alert tag)
Obtain emergent care for any new onset of pain and/or neurological systems
Proceed with caution - tissue fragility leads to tears, excessive bleeding
Manage hypertension, avoid constipation, carefully consider risks of pregnancy
Lifestyle:
Avoid contact sports and other high risk activities
Maintain ideal body weight
Collagen Disorders - Summary
• Collagen - main structural protein in body
• Many genetically distinct types of collagen
• all have triple helix structure
• polypeptide chains with large (Gly -X-Y)n domain
• post translational modifications and extracellular processing critical
• Type I collagen: Heterotrimer
• abundant in skin, bones and ligaments.
• mutations in associated genes cause autosomal dominant OI
• point mutations in glycine can causing structural collagen abnormality
can be more severe than haploinsufficincy (protien suicide)
• multiple severely affected children with normal parent can arise due to
germline mosaicism
• Type III collagen: Homotrimer,
• mutations in gene encoding tyope III collagen associated with Vascular
EDS (type IV EDS) characterized by vascular and tissue fragility
• Many types of EDS but classic EDS usually has skin and joint manifestations