N = 150
advertisement

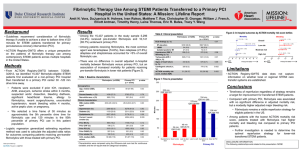
BRIGHAM AND WOMEN’S HOSPITAL A Prospective, Single-Arm, Multicenter Trial of UltrasoundFacilitated, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism (SEATTLE II) Gregory Piazza, MD, MS on behalf of the SEATTLE II Investigators March 30, 2014 Sponsored by the EKOS Corporation HARVARD MEDICAL SCHOOL TEACHING AFFILIATE Acute Pulmonary Embolism: A Spectrum of Risk 32% In-Hospital Mortality In-Hospital Death or Clinical Deterioration RV Dysfunction with +Troponin Unstable 3.4% In-Hospital Mortality RV Dysfunction OR +Troponin Stable Normal RV and Troponin Casazza F, et al. Thromb Res 2012;130:847 Becattini C, et al. CHEST 2013;144: 1539 Increased RV/LV Ratio on CT and PE-Related Mortality Trujillo-Santos J, et al. J Thromb Haemost 2013;11: 1823-1832 Intracranial Hemorrhage: Efficacy at the Cost of Safety Study ICOPER (Goldhaber SZ, et al. 1999) PEITHO (Meyer G, et al. 2014) Intracranial Hemorrhage (Fibrinolysis Group) 9/304 (3%) 10/506 (2%) Objectives A prospective, single-arm, multicenter trial to: • Evaluate the efficacy of ultrasound-facilitated, catheter-directed low-dose fibrinolysis to reverse RV dysfunction as measured by CT-determined RV/LV diameter ratio in patients with acute massive and submassive PE • Assess the safety of ultrasound-facilitated, catheter-directed low-dose fibrinolysis in patients with acute massive and submassive PE Patient Selection Main Inclusion Criteria: Main Exclusion Criteria: • Proximal PE on CT (filling defect in ≥ 1 main, lobar, or segmental pulmonary artery) AND • • Age ≥ 18 years AND • • • PE symptom duration ≤ 14 days AND • Massive PE (syncope, systemic arterial hypotension, cardiogenic shock, or resuscitated cardiac arrest) OR • Submassive PE (RV/LV diameter ≥ 0.9 on contrast-enhanced chest CT) • • • • • Stroke/TIA, head trauma, or intracranial or intraspinal disease within 1 year Active or recent (within 1 month) bleeding from a major organ Major surgery within 7 days Hematocrit < 30%, platelets < 100k/μL, INR > 3, aPTT > 50 seconds on no anticoagulation Serum creatinine > 2 mg/dL Clinician-determined high-risk for catastrophic bleeding Hemodynamic instability despite medical therapy Pregnancy Study Overview CT-confirmed PE • Symptoms ≤ 14 days • Massive or submassive • Meets all inclusion and no exclusion criteria RV enlargement as documented by initial CT • RV:LV ratio ≥ 0.9 Ultrasoundfacilitated fibrinolysis • t-PA 1 mg/hr for 24 hours (1 device) • t-PA 1 mg/hr for 12 hours (2 devices) • TOTAL t-PA Dose = 24 mg Study Sites = 21 Total Trial Population = 150 Follow-up at 48 ±6 hours after start of the procedure • CT measurement of RV:LV ratio • Echocardiogram to estimate PA systolic pressure Intervention Standard Anticoagulation for PE UFH goal PTT 40-60 sec during procedure Catheter Placement and Treatment Based on Extent of Disease Unilateral: 1 catheter infusing t-PA 1 mg/hour for 24 hours Bilateral: 2 catheters infusing t-PA 1 mg/hour/catheter for 12 hours Baseline Right Heart Catheter Measurements Including pulmonary artery systolic pressure Ultrasound-Facilitated, Low-Dose, Catheter-Directed Fibrinolysis t-PA Infusion Activation of high frequency, low power ultrasound Monitoring in intermediate care or ICU setting Procedure Completion Post-Procedure Right Heart Catheter Measurements Catheter Removal Study Outcomes • Primary Efficacy: Change in core labmeasured RV/LV ratio from baseline to 48 hours as assessed by chest CT • Primary Safety: Adjudicated major bleeding within 72 hours of the start of the procedure • Secondary Efficacy: Change in invasively measured PA systolic pressure from baseline to device removal and as estimated on 48-hour echocardiogram • Secondary Safety: Adjudicated recurrent PE or death within 30 days of the procedure, or major technical procedural complications Patients Study Enrollment Baseline Characteristics Patient Demographics Mean age ± SD, years Mean BMI ± SD, kg/m2 Female gender , n (%) Race/Ethnicity, n (%) Caucasian African American Hispanic Co-morbid Conditions, n (%) Concomitant use of antiplatelet agents Immobility within 30 days of PE Diabetes mellitus Previous DVT Previous PE N = 150 59 ± 16.1 35.6 ± 9.1 77 (51.3) 119 (79.3) 22 (14.7) 9 (6) N = 150 52 (34.7) 45 (30) 42 (28) 30 (20) 15 (10) Characteristics of PE Characteristics of PE, n (%) Duration of symptoms ≤14 days >14 days Any symptoms of PE PE subtype Submassive Massive Pre-procedure anticoagulation* Intravenous unfractionated heparin Enoxaparin Warfarin Other None *Patients could have received more than one anticoagulant. N = 150 149 (99.3) 1 (0.7) 150 (100) 119 (79.3) 31 (20.7) 76 (50.7) 54 (36) 16 (10.7) 7 (4.7) 24 (16) Procedural Characteristics Procedural Characteristics Mean dose of t-PA ± SD*, mg Successful device placement**, n (%) Access sites***, n (%) Right femoral vein Left femoral vein Right internal jugular vein Other Number of devices per patient*, n (%) 0 1 2 Completed infusion of t-PA***, n (%) 23.7 ± 2.9 278 (97.5) 177 (63.7) 61 (21.9) 31 (11.2) 9 (3.2) 1 (0.7) 20 (13.3) 129 (86) 272 (97.8) *N = 150 patients (1 patient died before devices could be placed) **N = 285 devices attempted ***N = 278 devices placed Outcomes: RV/LV Ratio p < 0.0001 RV/LV Ratio 1.55 1.13 Outcomes: PA Systolic Pressure p < 0.0001 Mean PA Systolic Pressure (mmHg) 51.4 p < 0.0001 37.5 36.9 Massive vs. Submassive PE p = 0.31 p = 0.61 0.51 0.5 14.3 0.43 0.4 12.6 0.3 0.2 0.1 Massive PE 0 Submassive PE Mean Decrease RV/LV Ratio Outcomes: Modified Miller Score Mean Modified Miller Score p < 0.0001 22.5 15.8 Clinical Outcomes Clinical outcomes* Mean length of stay ± SD, days In-hospital death, n (%) 30-day mortality**, n (%) Serious adverse events due to device, n (%) Serious adverse events due to t-PA, n (%) IVC filter placed, n (%) Major bleeding within 30 days**, n (%) GUSTO moderate** GUSTO severe** Intracranial hemorrhage, n (%) N = 150 8.8 ± 5 3 (2) 4 (2.7) 2 (1.3) 2 (1.3) 24 (16) 17 (11.4) 16 (10.7) 1 (0.7) 0 (0) *All death, serious adverse, and bleeding events were adjudicated by an independent safety monitor. **N = 149 (1 patient lost to follow-up) Discussion • We observed a 30% decrease in CT-measured RV/LV ratio over 48 hours in patients with massive and submassive PE treated with ultrasound-facilitated catheter-directed low-dose fibrinolysis. • Ultrasound-facilitated catheter-directed low-dose fibrinolysis rapidly relieved pulmonary artery obstruction and reduced pulmonary hypertension. • Ultrasound-facilitated catheter-directed low-dose fibrinolysis minimized the risk of intracranial hemorrhage. Overcoming the Hurdle of Intracranial Hemorrhage Study ICOPER (Goldhaber SZ, et al. 1999) PEITHO (Meyer G, et al. 2014) SEATTLE II (Piazza G, et al. 2014) Intracranial Hemorrhage (Fibrinolysis Group) 9/304 (3%) 10/506 (2%) 0/150 (0%) Limitations • The single-arm study design precluded direct comparison with the efficacy and safety of systemic fibrinolysis or anticoagulation alone. • Our study design did not allow for evaluation of clinical end points such as hemodynamic collapse or mortality. Conclusions • Ultrasound-facilitated catheter-directed low-dose fibrinolysis for acute PE improves RV function and decreases pulmonary hypertension and angiographic obstruction. • By minimizing the risk of intracranial bleed, ultrasound-facilitated catheter-directed low-dose fibrinolysis represents a potential “gamechanger” in treatment of high-risk PE patients. SEATTLE II Investigators • • • • • • • • • • • • Gregory Piazza, M.D., M.S. Tod C. Engelhardt, M.D. Keith M. Sterling, M.D. Noah J. Jones, M.D. John C. Gurley, M.D. Rohit Bhatheja, M.D. Robert Kennedy, M.D. Nilesh Goswami, M.D. Kannan Natarajan, M.D. John Rundback, M.D. Immad Sadiq, M.D. Stephen K. Liu, M.D. • • • • • • • • • • • Narinder Bhalla, M.D. M. Laiq Raja, M.D. Barry S. Weinstock, M.D. Jacob Cynamon, M.D. Fakhir F. Elmasri, M.D. Mark J. Garcia M.D. Mark Kumar, M.D. Juan Ayerdi, M.D. Peter Soukas, M.D. William Kuo, M.D. Samuel Z. Goldhaber, M.D. Special thanks to: •Benjamin Hohlfelder, B.S. (Thrombosis Research Group) Back-Up Slides Rationale for Study Design • Timely enrollment in randomized controlled trials for PE has historically been problematic. • A single-arm design allowed for efficient evaluation of the safety and efficacy of ultrasound-facilitated, catheter-directed low-dose fibrinolysis for treatment of PE. • Our study provided clinicians performing ultrasound-accelerated catheter-directed low-dose fibrinolysis with a standardized protocol for the procedure, which had been performed with little standardization among operators. • Our study design allowed for more rigorous monitoring and reporting of adverse events. Meyer G, et al. N Engl J Med 2014; in press Kucher N, et al. Circulation 2014;129:479