Applying CRASH2 Pre-hospital and Wilderness
advertisement

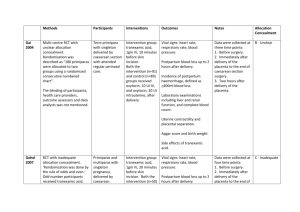
Applying CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2) in a PreHospital Wilderness Context Paul B. Jones PGY1 McMaster Family Medicine My question? Does the administration of tranexamic acid (TXA) among adult trauma patients with, or at risk of significant hemorrhage have an impact on death? Why do I care? Wilderness medicine, including the application of first-aid, includes any context that involves patient care in extreme environments, when resources may be limited or non-existent, and evacuation to greater medical care may be hours, days or longer. Applications of wilderness medicine may be in a remote corner of the planet, but also include environments such as urban disasters, severe weather conditions, multiple patients, police and military interventions or any situation that creates a context with minimal resources or extended scene patient management. A large randomised placebo controlled trial among trauma patients with, or at risk of, significant haemorrhage, of the effects of antifibrinolytic treatment on death and transfusion requirement Double blind RCT, London School of Hygiene and Tropical Medicine 274 Hospitals, 40 Countries, 20 211 adult trauma patients Adult trauma patients with significant haemorrhage Systolic blood pressure < 90 mm Hg or heart rate >110 beats per min, or both, or who were considered to be at risk of significant haemorrhage, and who were within 8 hours of injury. “Uncertainty principle” Patients with clear indication for tranexamic acid were not randomly assigned Patients with a clear contraindication for tranexamic acid were not randomly assigned “When the responsible doctor was substantially uncertain as to whether or not to treat with this agent these patients were eligible for randomization” Intervention Patients were randomly allocated to either: Treatment - receive a loading dose of 1 g tranexamic infuse over 10 min, followed by IV infusion of 1 g over 8 h. Placebo - matching (0.9% saline). Primary Outcome Death in hospital within 4 weeks of injury Cause of death categories: Bleeding Vascular occlusion (MI, CVA, and PE Multiorgan failure Head injury Other Secondary Outcomes Vascular occlusive events (MI, CVA, PE or DVT) Surgical intervention Receipt of blood transfusion and units of blood products transfused Dependency (dead, fully dependent day & night, or dependent but not needing constant attention) Baseline characteristics 1. Estimated hours since injury (<1, 1-3, 3-8h) 2. Systolic blood pressure (≤ 75, 76-89, 90 ≥ mm Hg) 3. GCS (severe 3-8, moderate 9-12, mild 13-15) 4. Type of injury (penetrating only or blunt, which included blunt and penetrating) Table 1 TXA vs. Placebo Table 2 Death by Cause 2x2 – Any Death Any Death No Death TXA 1463 (a) 8597 (b) Placebo 1613 (c) 8454 (d) CER = 0.16023 EER = 0.14543 RR = 0.90763 RRR = 0.09237 = 9.24% ARR = 0.0148 = 1.48% NNT = 67.57 2x2 – Bleeding Death Bleeding Death No Death TXA 489 (a) 9571 (b) Placebo 574 (c) 9493 (d) CER = 0.05702 EER = 0.04861 RR = 0.85251 RRR = 0.14749 = 14.75% ARR = 0.00841 = 0.84% NNT = 118.91 All-cause mortality by subgroups All-cause mortality by subgroup Cochrane Review “Each year, world-wide, about four million people die as a result of traumatic injuries and violence. Approximately 1.6 million of these deaths occur in hospital and about one third of these deaths (480,000) are from haemorrhage. The CRASH-2 2010 trial has shown that TXA reduces mortality from haemorrhage by about one sixth. If this widely practicable intervention was used world- wide in the treatment of bleeding trauma patients, it could prevent over 70,000 deaths each year.” (Antifibrinolytic drugs for acute traumatic injury. Cochrane Database of Systematic Reviews 2011) Cochrane Review Tranexamic acid (TXA) safely reduces mortality in bleeding trauma patients. Because most deaths from traumatic haemorrhage occur in the first few hours after injury, every effort should be made to treat patients as soon as possible. More research on mortality and morbidity is required on the use of TXA in TBI patients before it can be recommended in this population. (Antifibrinolytic drugs for acute traumatic injury. Cochrane Database of Systematic Reviews 2011) References CRASH-2 trial collaborators (2010) Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebocontrolled trial. Lancet Jul 3;376(9734): 23–32. Guerriero C, Cairns J, Perel P, Shakur H, Roberts I, et al. (2011) CostEffectiveness Analysis of Administering Tranexamic Acid to Bleeding Trauma Patients Using Evidence from the CRASH-2 Trial. PLoS ONE 6(5): e18987. doi:10.1371/journal.pone.0018987 The CRASH-2 Trial Collaborators (2006) Improving the evidence base for trauma care: Progress in the international CRASH-2 trial. PLoS Clin Trials 1(6): e30. DOI: 10.1371/journal.pctr.0010030 Roberts I, Shakur H, Ker K, Coats T, on behalf of the CRASH-2 Trial collaborators. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No.: CD004896. DOI: 10.1002/14651858.CD004896.pub3. Gruen RL, Biswadev M. Tranexamic acid for trauma. Lancet. Published Online March 24, 2011 DOI:10.1016/S0140- 6736(11)60396-6