CLL - Imedex
advertisement

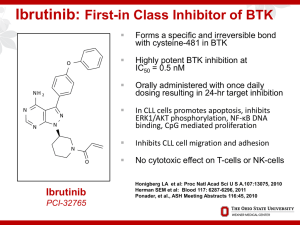
Hematology Highlights 2013 Expert Reviews of the Annual Hematology Meeting Chronic Lymphocytic Leukemia (CLL) Kanti R. Rai, MD NSLIJ-Hofstra School of Medicine Long Island Jewish Medical Center New Hyde Park, NY Agenda in CLL • Chemo-immunotherapy • Novel agents • Who should be referred for allogeneic SCT? Disclosures Member Medical Advisory Board – Genentech, Teva, Celgene, GSK, Sanofi Evolution of FCR in CLL Keating et al introduced FCR and its dramatic results in front line CLL Byrd et al (CALGB) introduced - FR. FCR - Keating et al JCO 2005;23:4079-4088, Blood 2008;112:975-980 FR - Byrd et al Blood 2003;101:6-14 FCR – Keating et al, Tam et al Single center Phase II Trial. N = 300 Median Age – 57 years Over 70 year of age were 14%. ORR 95% , CR 72 %. MRD Negative CR – 78% At 6 years OS 77% , FFS 51 % 6 year survival : MRD Negative vs Positive : 84% vs 65%. FCR-300 Survival and Time to Fail OS 1.0 0.8 Proportion 0.6 0.4 Pts. 300 300 0.2 Event 106 Survival 170 Time to Fail 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 Years Slide courtesy Dr Michael Keating FCR vs FC (CLL8 Trial) Hallek et al Lancet 2010;376:1164 Phase III International Randomized study N=817 Median Age = 61 years Median follow up 3.5 years FCR Arm FC Arm • ORR/CR - 90/44* • ORR/CR - 80/22 # • OS 84 % • OS 79 % # # * cf Keating CRs 72% # P<0.001 ## P = 0.01 FCR vs FC Phase III Trial GCLLSG Overall Survival At 3 years, 87 % of patients in the FCR group were alive vs. 83% in the FC group (HR- 0·67 [95% CI 0·48–0·92], p<0·01) Hallek et al Lancet 2010 Bendamustine with Rituximab (BR) by GCLLSG Fischer et al : Multicenter Phase II (JCO 2012) N=117 Median age 64 years OR/CR – 88/23.1 % CLL 10 trial comparing FCR and BR is closed now. FCR vs BR – an overview Author Number of patients CR OR Hallek et al 817 44/22 (FCR/FC) 90/80 (FCR/FC) Keating et al Tam et al 300 72 95 Byrd et al (FR) 104 47/28 (FCRconcurrent/ FCR Sequential) 90/77 23 88 Fischer et al (BR) 117 FCR vs BR – an overview FCR (German) BR (German) FCR (MDACC) Anemia 22 (5%) 23(19.7%) - Thrombocytopenia 30 (7%) 26(22.2%) 5(2.2%) Infections 103 (25%) 9(7%) 2.6% of courses Age >65 (n/CR%) (54/43) (26/3) (30/47) Other variants of FCR FCR lite - Foon et al JCO 2009, Blood March 2012. Sequential F-C-R - Lamanna et al JCO 2009 FCR with Alemtuzumab (CFAR) –Wierda et al Blood 2011 FCR with mitoxantrone (R-FCM) –Bosch et al JCO-2009 Len-Rituximab Single agent Lenalidomide is active in elderly patients. Phase II study – n=59 ,RR CLL Rituximab (375 mg/m2) weekly C1 and on day 1 of C3-C12. Lenalidomide was started on day 9 of C1 at 10 mg daily continuously in 28 day cycles. Rituximab was administered for 12 cycles. ORR - 66% (12%-CR). TTF (17.4 months). Median OS (NR) estimated survival at 36 months is 71%. Grade 3/4 toxicity - neutropenia (73%). Grade 3/4 Infection or febrile episode (24%) Badoux et al JCO; Dec26th 2012 BCR Signaling pathway Choi M et al Cancer J 2012;18: 404-410 BCR signaling inhibitors Btk (Bruton tyrosine kinase) Inhibitor – Ibrutinib and AVL-292 PI3Kδ-p110 isoform inhibitor- GS-1101 and IPI-145 Syk (spleen tyrosine kinase inhibitor) – Fostamatinib, Portola compounds Lyn – Kinase inhibitor –Dasatinib, Bafetinib Ibrutinib Ibrutinib Promotes High Response Rate, Durable Remissions, and Is Tolerable in Treatment Naïve and Refractory CLL/SLL Including Patients with High-Risk (HR) Disease: Updated Results of 116 Patients in a Phase Ib/II Study. Abstract – 189, Byrd J. et al Btk Inhibitor (Ibrutinib) Bruton like tyrosine kinase (Btk) is a downstream mediator of B-cell receptor (BCR) signaling and is not expressed in T-cells or NK-cells. Oral drug (420 mg qd), irreversible Btk inhibitor. N=116, Relapsed refractory CLL(n=61) vs frontline (n=31; all age >65 yrs). ORR 67 % vs 71%, well tolerated. 22 months PFS – 76% and 96%. Combination trials with Ofatumumab, FCR or BR are ongoing. Byrd J et al ASH 2012 Btk Inhibitor (Ibrutinib) with Rituximab Ibrutinib 420 mg PO daily, in combination with weekly rituximab (375 mg/m2) for weeks 1-4 (cycle 1), then daily ibrutinib plus monthly rituximab until cycle 6, followed by daily single-agent ibrutinib. 17/20 pts – ORR 85% in high risk patients Shorter redistribution Lymphocytosis due to Rituximab Burger JA et al ASH 2012 Idelalisib (GS-1101) PI3K p110 δ isoform inhibitor. Oral drug (150 mg po bid). N=54, relapsed refractory CLL. ORR 33% (all PR) and LN response in 100% cases. Pneumonia and colitis 24% Significant effect on lymphocyte trafficking and redistribution. Combination trials with lenalidomide, Rituximab and Bendamustine are ongoing. Furman RR et al ASCO 2012 Idelalisib Combined With Ofatumumab Substantially Increased Overall Response Rate GS-1101 Mono GS-1101 + O (N=55) 100 Responsea Rate +95% CI 80 85% n=17 84% n=46 60 80% n=16 94% n=15 40 20 24% n=13 CR 10% CR 6% LNR OR OR (N=20c) (N=20) 0 Lymph Node Overall Responsea Responseb (LNR) (OR) 6 cyclesd (N=16) Decrease by 50% in the nodal SPD Response as assessed by investigators based on IWCLL criteria (Hallek 2008) C 1 Subject without follow-up assessment was excluded from analysis d Subjects having received 6 cycles of therapy a b Furman RR et al ASCO 2012 Idelalisib (GS-1101) with BR Combinations of PI3Kδ inhibitor GS–1101 with Rituximab (R) and/or Bendamustine (B) Are Tolerable and Highly Active in Patients with RR CLL: Results From a Phase I Study Abstract – 191, Coutre SE et al Idelalisib (GS-1101) with BR GS-1101 with R or with B or with both BR. GS-1101 dose of 150 mg/dose BID orally. ORR for the GS-1101/R, GS-1101/B, and GS-1101/BR regimens were 78%, 82% and 87%. With a minimum follow-up of 40 weeks, 1-year PFS rates were 74%, 88% and 87% in the GS-1101/R, GS-1101/B, and GS-1101/BR respectively. Adverse effects were common with GS-1101/B arm. Abstract – 191, Coutre SE et al # Indications of allo SCT in CLL Young and physically fit patients with Richter’s transformation Refractory patients with del17p or TP53 mutations Relapsed patients with fludarabine refractory disease Ultra High risk patients with CLL #These indications may change after the approval of BCR inhibitors for the therapy of CLL CLL Collaborations CLL Research Consortium (CRC) NCI- Working Group on CLL International Workshop on CLL (iwCLL) German CLL Study Group CLL Global Research Foundation Alliance for Clinical Trials in Oncology (CALGB)