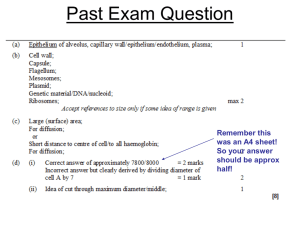
• The lung function starts with the first moment of life and ceases with
death.
• In an intermediate period in females life journey, hormonal changes
start with the menarche and ends by the menopause.
• It seems that the lungs are affected by such biological feminine events.
• It had been observed through centuries by many women that some
respiratory symptoms and even distinct clinical morbidities associate
with their cycles.
• Emerging understanding of the role of sex hormones in respiratory
health and disease represents a major advance in personalized
treatment planning for menses associated respiratory aliments.
Early menses lead to asthma, poor lung function
Women who start menstruating early are at a high risk of developing asthma and
poor lung function.
The study suggests that women with early menarche have lower lung function and
more asthma risk in adulthood reaffirming the role of hormonal and metabolic
factors in women's respiratory health.
American Journal of Respiratory and Critical Care
Medicine, August 2010
• Respiratory symptoms during
menstruation
• Catamenial lung disorders
Women with asthma experience
cyclic changes in airflow as well as
gas transfer and membrane
diffusing capacity supportive of a
hormonal effect on lung function.
• SOB – TIC – Chest pain
• Exacerbation / deterioration
of already present lung
diseases
• Pulmonary endometriosis
• Endometrial tissue is located in the pleura, lungs bronchi and or the
diaphragm
• Endometrial cells at these sites are affected by the hormonal changes of the
menstrual cycle with concomitant active bleeding
• The clinical presentations include :
Catamenial pneumothorax 80%
Catamenial haemothorax
14%
Catamenial haemoptysis
5%
Lung nodules
rare
Thoracic endometrial syndrome was first described by Barnes J in 1953 in J Obst.
Gyncolog. Br. Emp : Endometriosis of the pleura and ovaries 60(6) : 823-24
Between 2:10 % of females at reproductive
age world wide have Endometriosis
In USA between 5.5:6 Million females suffer
from Endometriosis
• Baron Carl von Rokitansky
1804 – 1878
• Austerian physician,
pathologist, humanist,
philosopher and liberal
politician
• 1st to describe systemic
Endometriosis
1. Open communication
between the atmosphere
and peritoneal cavity
during menstruation can
allow air to migrate from
the abdomen via
diaphragmatic
fenestrations into the
pleura. (cure achieved by
tubal and fenestration
obliteration)
2. Endometrial implants over the diaphragm induce the
defects
3. PGF2 excessively released during menstruation causes
bronchiolar and alveolar constriction and rupture
PGF2
4. Lymphatic & or haematogenous embolization
endometrial tissue from the uterine vessels
Women with bronchopulmonary
endometriosis tend to have a history
of uterine manipulation or trauma
(e.g., hysteroscopy, dilation and
curettage). This supports the
lymphovascular embolization theory,
whereas those with pleural disease
most often have a history of pelvic
endometriosis.
of
Concerning the etiology of CPT, it is hypothesized that transgression or
erosion of the diaphragm as an anatomic boundary by endometriotic
tissue represents the central pathophysiologic mechanism of CPT. This
can be stimulated through a heat-stable factor from the
peritoneal fluid, together with an increased proteolytic
capacity. Endometriotic cells can demonstrate a higher
maneuverability with an enhanced potential for local invasiveness
Catamenial Pneumothorax
Lillington and associates
coined the term
catamenial
pneumothorax. They
proposed a model in
which the expansion of
intraparenchymal
subpleural endometriotic
tissue during menses
would cause a checkvalve airway obstruction,
eventually leading to
alveolar rupture.
Lillington GA, Mitchell SP, Wood GA.
Catamenial pneumothorax. JAMA. Mar
6 1972;219(10):1328-1332.
Clinical picture of catamenial pneumothorax
Patients with CP present with
symptoms of spontaneous
pneumothorax that are usually
nonspecific such as :
1.
2.
3.
4.
Pleurisy,
Cough,
Shortness of breath
Peri-scapular or radiating
neck pain due to
diaphragmatic irritation.
In most cases, symptoms are mild
to moderate while severe
presentations are rare
Alifano M, Trisolini R, Cancellieri A, Regnard JF.
Thoracic endometriosis: current knowledge. Ann
Thorac Surg. Feb 2006;81(2):761-769.
Catamenial Pneumothorax
Endoscopy
In the largest review of CP cases,
more than 50% (52.1%) of patients
with CP assessed with VATS were
diagnosed as having thoracic
endometriosis. Diaphragmatic
abnormalities (fenestrations or
endometriosis, alone or combined)
are the most commonly described
lesions (38.8%), followed by
endometriosis of the visceral pleura
(29.6%). In the remainder of cases,
discrete lesions, such as bullae, blebs,
and scarring (23.1%), or no findings
(8.5%) are noted.
Korom S, Canyurt H, Missbach A, et al. Catamenial pneumothorax revisited: clinical
approach and systematic review of the literature. J Thorac Cardiovasc Surg. Oct
2004;128(4):502-508.
Catamenial Pneumothorax
Endoscopy
Diaphragmatic fenestrations range from a few millimeters to 2 cm. Endometrial deposits
in both the diaphragm and pleura have a similar appearance and range from a few
millimeters to 1 cm. Their color ranges from violet to brown, depending on the day of
menstrual cycle.
Performance of a combined VATS and laparoscopy procedure in a single session is
another diagnostic approach.
Alifano M, Venissac N, Mouroux J. Recurrent pneumothorax associated with thoracic
endometriosis. Surg Endosc. Jul 2000;14(7):680.
Catamenial haemothorax
CHT is an uncommon
manifestation of TES accounting
for approximately 14% of cases.
As with CP, CHt is almost always
unilateral and right sided,
although left-sided hemothorax
has been reported. Again,
symptoms are nonspecific and
include pleuritic pain, shortness
of breath, and cough. The
presence of bloody effusion is
variable. Computed tomography
(CT) of the chest may show
multiloculated effusions, nodular
lesions of the pleura, or bulky
pleural masses.
Catamenial haemoptysis and lung nodules
CH and lung nodules are both clinical
entities of bronchopulmonary TES and
are very rare manifestations.
Hemoptysis is a quite variable
manifestation, with neither massive
hemoptysis nor deaths being described
so far. An association with menses may
not always be appreciated, and
diagnostic delays of up to 4 years from
the onset of symptoms have been
reported. CH and lung nodules are
interrelated entities. Thus, patients
who present with CH frequently have
associated lung nodules on imaging
studies and vice versa.
CP, CHt, CH, and lung nodules represent
the main clinical entities in TES.
However, they are not the only
manifestations of TES, other
manifestations include catamenial
phrenic nerve irritation causing a
catamenial pain-only syndrome, namely
cyclic shoulder, neck, epigastric, or right
upper quadrant pain
Imaging in Thoracic Endometriosis
Imaging in Thoracic Endometriosis
X ray chest and preferably CT scan can identify menstrual associated pulmonary and
bronchial infiltrates and confirm both their amelioration by the end of the cycle and their
recurrence with each following cycle. Thus performing imaging studies and bronchoscopy
during menses assist in the diagnosis of pleural and bronchopulmonary disease. i.e
Repeated imaging studies or bronchoscopy during midcycle typically documents the
disappearance of the previously reported findings, thus strengthening the clinical
suspicion.
Hope-Gill B, Prathibha BV. Catamenial haemoptysis and clomiphene citrate
therapy. Thorax. Jan 2003;58(1):89-90.
Treatment of Catamenial Pneumothorax
VATS is the gold standard modality for both the definitive diagnosis and surgical
treatment of CP.
Alifano M, Trisolini R, Cancellieri A, Regnard JF. Thoracic endometriosis: current
knowledge. Ann Thorac Surg. Feb 2006;81(2):761-769.
Tissue diagnosis of respiratory
endometriosis can be achieved by:
1. FOB forceps biopsy
2. TBNA
3. US/CT guided lung biopsy
4. Surgical lung biopsy
Medical Treatment
1.Danazol
2.Contraceptive pills
3.GnRH analogues
Recurrent rate at 1 y :
50 – 60 %
Medical Treatment often serves as a
diagnostic tool with the +ve response
paving the way for more effective
surgical treatment
1.Thoacentesis and chest tube
2.VATS +/- laparoscopy : with complete inspection of the pleura
and both diaphragmatic surfaces for fenestrations and nodules
3.Small, few mms, endometrial nodules can be fulgurated by
diathermy or CO2 laser
4.Large nodules should be excised from the pleura and the lungs
even if combined with necessary parenchymal resection :
segmentectomy or lobectomy
5. Large diaphragmatic fenestrations can be sutured +/- mesh
coverage
6. Pleurodesis in combination with any of the previous
procedures adds to the efficiency of management
7. Based on the recurrence rate estimates of pervious modalities
combined surgical and subsequent hormonal treatment is
recommended
TES is a challenging
clinical entity. A high
index of clinical
suspicion is of
paramount importance
as both diagnosis and
treatment may often
be delayed for years.
Endometriosis has variable and often subtle
clinical and macroscopic features that include
Catamenial pneumothorax
Catamenial haemothorax
Catamenial haemoptysis
Lung nodules
A multidisciplinary approach by
thoracic and gynecologic surgical
teams carries the highest chance
of making an accurate diagnosis
and providing the appropriate
treatment strategies.