MariellaGarcia
advertisement

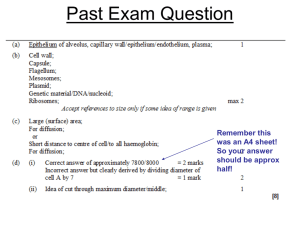
ALK fusion and MET amplification as molecular markers and therapeutic targets in advanced lung adenocarcinomas Marileila Varella Garcia, PhD On behalf of the Lung Cancer Mutation Consortium Investigators Grand Opportunity Grant- NCI 1 RC2 CA148394; PI Paul Bunn Lung Cancer Mutation Consortium • Define molecular status of 10 relevant genes in 1000 advanced lung adenocarcinomas – Activation of KRAS, EGFR, BRAF, HER2, PIK3CA, AKT1, NRAS, MEK tested by mutation analyses (Johnson, O16.01) – Activation in ALK (gene fusion) and MET (amplification) tested by FISH analyses • Assist in the implementation of qualified molecular diagnostic laboratories • Identify personalized therapy with approved/trial agent targeting specific mutations Lung Cancer Mutation Consortium 14 US Institutions Study Preliminary Conclusions Protocols and reagents for 10 markers were optimized High quality molecular diagnosis for multiple markers was achieved in a period of time reasonable to select patients for target therapy Turn Around Time for 2 FISH tests was <1 week Turn Around Time for all 10 tests was ~2 weeks Current panel of mutations may be easily optimized for increased number of driver mutations Actionable driver mutations were detected in 4.1% of adenocarcinomas in MET (amplification) and in 9.6% in ALK (rearrangements) 90.4% ALK- N=643 9.6% ALK+ 94.9% MET- N=295 4.1% MET+ 0 20 40 60 80 ~55% adenocarcinomas carry mutations for one of 10 markers tested (Johnson, O16-01) 100 ALK positive patients are sensitive to crizotinib Significant fraction of ALK+ patients were enrolled in crizotinib trials with excellent response Some progressed in crizotinib and are now considered for other clinical trials MET amplified patients are resistant to EGFR TKI inhibitors and sensitive to MET inhibitors Some MET+ patients were enrolled in the Pfizer A8081001 with good response Efforts are ongoing to enroll MET+ patients in other trials with specific MET inhibitors Conclusions ALK fusions and MET amplification are present in significant fraction of lung adenocarcinomas Identification of tumors with actionable driver significantly improves clinical outcome when patients are treated with specific inhibitors FISH technology is a robust platform for testing biomarkers in lung tumors within a short turn around time and low failure rate Collaborative efforts are essential to qualify laboratories for molecular testing in a cost-effective and efficient manner The Lung Cancer Mutation Consortium study sponsored by NCI represents a successful model for translational science in lung cancer