Meeting the Challenge of Mandatory HAI Reporting
advertisement

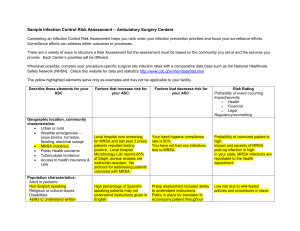
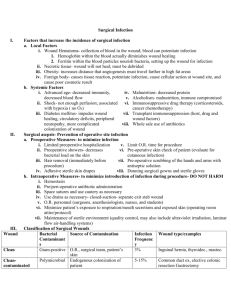
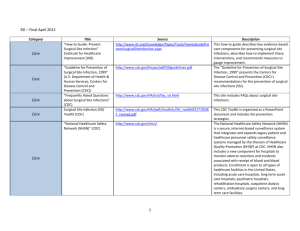
Meeting the Challenge of Mandatory HAI Reporting Marcy Maxwell RN, BSN, CIC Dignity Health March 6, 2012 Objectives • Provide broad overview of forces leading to development of current requirements • Review current HAI reporting mandates for California and CMS • Share Dignity’s Health success in meeting surgical procedure reporting Institute Of Medicine 1999 • Preventable medical errors in hospitals exceed attributable deaths to such feared threats as motorvehicle wrecks, breast cancer, and AIDS. • Identify and learn from errors by developing a nationwide public mandatory reporting system and by encouraging health care organizations and practitioners to develop and participate in voluntary reporting systems. • State governments will be required to collect standardized information about adverse medical events that result in death and serious harm. Institute for Healthcare Improvement 2004 • "Some Is Not a Number, Soon Is Not a Time” • Campaign with specific commitment to produce measurable results in quality within a time certain, on a national scale, including – Reliable use of central venous line bundles – Surgical site infection prophylaxis – Reliable use of ventilator associated pneumonia bundles Reporting Policy Fundamentals • Must report via National Healthcare Safety Network (NHSN) – database developed and maintained by the CDC • Use of standardized definitions to allow meaningful comparison • Hospital data publicly reported • Rates of infections will be used for CMS reimbursement- 2012 reporting linked to 2014 payments State and National Requirements are not the same! CMS- Req’t Began CMSLocations CDPH- Req’t Began CDPHLocations CLIP - - 2008 ICU/NICU MRSA -BSI 2013 All 2009 All VRE- BSI - 2009 All CDI 2013 All 2009 All CLABSI 2011 ICU/NICU/PICU 2010 All Surgical Procedures 2012 2 procedures 2011 29 procedures CAUTI 2012 ICU/PICU - 6 SB 739- 2008 • Submit process measure data to CDPH utilizing CDC definitions • Report central line bloodstream infection rates • Report central line insertion practices (CLIP) • Influenza vaccination rates of patients and employees Nile’s Law 2009 • MRSA screening on admission and discharge • All cases of health-care-associated MRSA bloodstream infection, clostridium difficile infection, and VRE bloodstream infection and number of inpatient days • All central line associated bloodstream infections and the total central line days • All health care associated surgical site infections of deep or organ space surgical sites, health care associated infections of orthopedic surgical sites, cardiac surgical sites, and gastrointestinal surgical sites • ( or so CDPH thought!....) California Hospital Association Files Lawsuit to Halt New Surgical Site Infection Reporting Requirements • The California Hospital Association (CHA) has filed a lawsuit against the California Department of Public Health (CDPH) to stop the implementation of new public reporting requirements for surgical site infections (SSI). The lawsuit was filed Thursday, May 26, 2011. • At issue are SSI public reporting requirements mandated by the CDPH in the state’s revised All Facilities Letter (AFL) No. 1132 which was released on April 26, 2011. As stated in the AFL, the CDPH now requires hospitals to collect and report data on 29 different surgical procedures and any resulting SSIs to the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network (NHSN 9 SSI Denominator Requirements- 2011 • 29 NHSN codes, approx. 200 ICD-9 codes • Requires detailed information on each procedure- minimum 15 data elements • Must report via NHSN • Data publicly reported and used for CMS reimbursement • 2012 reporting linked to 2014 payments 10 Resistance is Futile! 11 Dignity Health Response • Development of the SSIR- Surgical Site Infection Reporting application – Web-based application – Identifies reportable procedures based on ICD-9 codes – Up to half of required data elements available from registration, ADT data – For facilities with surgery systems, flat file is run monthly and uploaded into SSIR; merged with registration data by medical record number, date of procedure. • When all records completed, file is saved, uploaded to NHSN. 12 SSIR The SSIR has significantly reduced manual data entry by IPs, while accurately identifying reportable procedures. 13 Summary- The Good News • Goal set in 1999 to create a nationwide public mandatory reporting system using standardized definitions has been realized. • Financial (dis)incentives, public reporting and consumer interest have increased the visibility of infection prevention. • There has been a significant shift in attitude re: HAI prevention, good for patient safety. • Automated systems for meeting data requirements are possible. On-Going Challenges • Time spent meeting reporting requirements comes at the expense of other infection prevention activities. • Too early in the process to determine the effect of reporting on rates of HAI. • Insufficient to simply report infections, the purpose is to prevent/reduce infections and increase patient safety. • Basic hand hygiene and environmental sanitation remains the key to reducing HAIs. 15 Thank You