Anesthetic Implications of
End-Stage Liver Disease and
Liver Transplantation
Todd M. Oravitz, MD
Associate Professor
Department of Anesthesiology
University of Pittsburgh School of Medicine
Chief, Liver Transplantation Anesthesia
VA Pittsburgh Healthcare System
Lecture objectives
1) Discuss the pathophysiology of end-stage liver
disease
2) Discuss the management of anesthesia in
patients with end-stage liver disease
3) Discuss the perioperative management of
patients undergoing liver transplantation
4) Discuss the perioperative management of
patients undergoing procedures after liver
transplantation
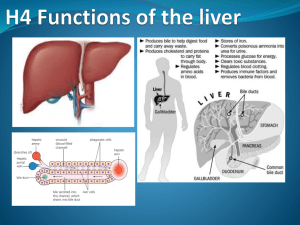
Normal Hepatic Function
• Liver plays a role in
– Carbohydrate metabolism
• Produces/stores glycogen, which can be
depleted after 24-48 hours of fasting
• Site of gluconeogenesis, with amino acids,
glycerol and lactate as substrates
Normal Hepatic Function
• Liver plays a role in
– Protein metabolism
• All plasma proteins, except for
immunoglobulins, made in the liver
– Albumin helps maintain plasma oncotic
pressure and is the primary binding/transport
protein for many anesthetic drugs
• All coagulation factors, except for factor
VIII and von Willebrand factor, made
in the liver
Normal Hepatic Function
• Liver plays a role in
– Drug metabolism
• Most medications undergo at least some hepatic
degradation or biotransformation, or both
• End products either metabolically inactive or more
water-soluble for biliary or urinary excretion
Normal Hepatic Function
• Drug metabolism
– Phase I reactions
• Include oxidation/reduction (redox)
• Cytochrome p450
• Benzodiazepines and barbiturates degraded via phase I
– Phase II reactions
• May or may not follow phase I
• Involve conjugation to facilitate elimination
via bile or urine
Normal Hepatic Function
• Drug metabolism
– Cytochrome p450
• Ethanol, ketamine capable of enzyme induction,
resulting in tolerance to the drugs’ effects
• Cimetidine, chloramphenicol can cause prolongation
of drug effects by enzyme inhibition
Normal Hepatic Function
• Anatomy/physiology
– Largest organ in the body, weighing about 1.5kg
– Right upper quadrant location
– Dual blood supply
• Liver blood flow ~1.5L/min
• Hepatic artery
• Portal vein
Normal Hepatic Function
• Dual blood supply
– Hepatic artery
• Accounts for 25% of blood flow and 50% of O2 delivery
• Flow is auto-regulated
– Portal vein
• Accounts for 75% of blood flow and 50% of O2 delivery
• Flow depends on GI and splenic blood flow
End-Stage Liver Disease (ESLD)
• The liver has a remarkable capacity
for regeneration
• The liver has tremendous physiologic reserve
• Hepatic disease can develop insidiously and
a large proportion of function can be lost
before problems become apparent
End-Stage Liver Disease (ESLD)
• Common symptoms
– Anorexia
– Weakness
– Nausea/vomiting
– Abdominal pain
• Common signs
– Hepatosplenomegaly
– Ascites
– Jaundice
– Spider angiomas
– Encephalopathy
Pathophysiology of ESLD
• Hepatic changes
– Portal hypertension – high resistance to blood
flow through the liver – hallmark of ESLD
– Leads to accumulation of blood and increased
venous pressure in the vascular beds
“upstream” to the liver
• Esophagus
• Spleen
• Stomach and intestines
Pathophysiology of ESLD
• Portal hypertension leads or contributes to
– Ascites
– Esophageal varices
– Gastric and other intra-abdominal varices
– Splenomegaly
Pathophysiology of ESLD
• Esophageal varices
– Portal-systemic collaterals that allow splanchnic
venous blood to flow from the high-pressure
portal system to the low-pressure azygos and
hemi-azygous system
– Not all patients with ESLD develop varices and
not all patients with varices have bleeding
– Patients that do bleed have significant
morbidity and mortality – up to 30%
of initial episodes of bleeding are fatal
Variceal Disease
• Treatment
– Chronic
• Propranolol is a non-selective beta-blocker
that decreases portal venous pressure
– Reduces risk of primary bleeding
– Reduces risk of re-bleed
• Banding, ligation, sclerotherapy
• Transjugular intrahepatic portosystemic shunt (TIPS)
Pathophysiology of ESLD
• TIPS
– Improves blood flow through the liver
– Percutaneous approach to create a shunt
between the portal and hepatic veins
– Decreases activity of the sodium-retaining
pathways
– Improves renal response to diuretics
TIPS
Variceal Disease
• Treatment
– Acute
•
•
•
•
•
•
Aggressive fluid resuscitation; ± blood
Correct coagulation defects, if present
Airway protection – intubation
Octreotide – reduces portal pressure
Endoscopy with possible intervention – banding
Balloon tamponade – Blakemore tube
Pathophysiology of ESLD
• Hepatic changes
– Spontaneous bacterial peritonitis (SBP)
• Spontaneous infection of ascitic fluid without
an intra-abdominal source
• Increased intestinal wall permeability allows
translocation of bacterial into the conducive
media of ascitic fluid
Pathophysiology of ESLD
• Hepatic changes
– Spontaneous bacterial peritonitis (SBP)
• Cefotaxime is the antibiotic of choice for treatment as
it covers 95% of the offending flora, including the 3
most common – E coli, Klebsiella and pneumococcus
• Quinolone (e.g. ciprofloxacin) prophylaxis is indicated
after an initial episode as there is a 70% recurrence rate
in the 1st year and it has a beneficial effect on patient
survival
• Two year survival after SBP is less than 50%
Pathophysiology of ESLD
• Hepatic changes
– Hepatic encephalopathy (HE)
• Occurs when substances normally metabolized
by the liver accumulate due to its dysfunction
– Ammonia felt to be most important in HE patients
• Increased activity of inhibitory neurotransmitters
also may play a role
– Increased GABAergic tone
– Administration of the benzodiazepine antagonist
flumazenil often results in an improvement in
the mental status of HE patients
Hepatic Encephalopathy
• Often occurs after a precipitating event
– Increased ammonia level
• Large dietary protein load
• GI bleeding
• Azotemia
– Decreased hepatic perfusion
• Anesthesia and surgery with resultant hypotension,
hypoxemia and/or hypovolemia
• Diuretic administration, paracentesis or GI
disturbance such as diarrhea or vomiting
Hepatic Encephalopathy
• Other possible precipitating events
– Sepsis
• Increased ammonia levels due to protein catabolism
• Decreased hepatic perfusion
– Creation of portal-systemic shunt
• TIPS
• Results in decreased hepatic metabolism
Hepatic Encephalopathy
• Treatment
– Remove/minimize, to the extent possible,
any/all underlying causes
– Decrease blood ammonia levels
• Reduce production
– Lower dietary protein intake
– Neomycin – targets urease-producing bacteria
• Reduce GI absorption
– Lactulose – non-absorbable disaccharide that
decreases large intestinal absorption of
ammonia and also promotes growth of
non-urease producing bacteria
Pathophysiology of ESLD
• Coagulation/hematologic changes
– Coagulopathy results mostly from two factors
• Impaired synthesis of clotting factors
• Thrombocytopenia
– Decreased levels of anticoagulants, most
notably antithrombin III and protein C,
can lead to thrombotic complications
• Portal vein thrombosis
• Deep venous thrombosis (DVT)
• Pulmonary embolism (PE)
Coagulation/Hematologic Changes
• Coagulopathy
– Impaired synthesis of coagulation cascade
proteins
• All clotting factors, except von Willebrand factor,
made in the liver
• Vitamin K dependent factors – II, VII, IX and X –
at additional risk
– Bile salts needed for intestinal absorption of vitamin K
and may be decreased by ESLD
– Overall poor nutritional status in many
ESLD patients
Coagulation/Hematologic Changes
• Coagulopathy
– Thrombocytopenia
• Portal hypertension-induced splenomegaly
– Occurs in 30-60% of ESLD patients
– Up to 90% of platelets can be sequestered
in the enlarged spleen
– Platelet count usually >30K and spontaneous bleeding
is fairly uncommon
• Associated disease processes can contribute
– Poor nutrition – folate deficiency
– Chronic alcohol intake
Pathophysiology of ESLD
• Cardiovascular changes
– Hyperdynamic circulation
•
•
•
•
•
Increased cardiac output
Decreased systemic vascular resistance
Normal to decreased blood pressure
Increased heart rate
Normal to increased stroke volume
Pathophysiology of ESLD
blood pressure=cardiac output x systemic vascular resistance
↔/↓BP = ↑CO X ↓SVR
↓
−−−−−−−−−−−−−−−−−
↓
↓
↑HR X ↔/↑SV
cardiac output = heart rate x stroke volume
Pathophysiology of ESLD
• Cardiovascular changes
– Result from development of vasodilation
and abnormal shunting
• Blood passes from the arterial to the venous
circulation without crossing a capillary bed;
an anatomic example of this is a spider angioma
• Thought to result from increased plasma levels of
glucagon and vasoactive intestinal polypeptide
Pathophysiology of ESLD
• Pulmonary changes
– Hypoxemia, with PaO2 values of 60-70mmHg,
is commonly seen in ESLD patients
– Causes include
•
•
•
•
Underlying cardiopulmonary disease
Intrapulmonary shunting
V/Q mismatch
Decreased diffusion capacity
Pulmonary Changes - Hypoxemia
• Underlying cardiopulmonary disease
– Congestive heart failure, interstitial lung disease,
chronic obstructive pulmonary disease
• Intrapulmonary shunting
– Pre-capillary or larger arteriovenous
communications are the result of
intrapulmonary vascular dilatation
– Hepatopulmonary syndrome
Hepatopulmonary Syndrome (HPS)
• Defined by the clinical triad of
– Chronic liver disease
– Increased A-a gradient
– Evidence of intrapulmonary vascular dilatation
• Increased pulmonary nitric oxide production
is the likely cause
• Usually diagnosed by echocardiography
Hepatopulmonary Syndrome (HPS)
• Incidence 5-30%
• Decreased survival compared to patients
with similar degree of liver disease who
do not have HPS
• HPS patients with severe preoperative
hypoxemia (PaO2 <50mmHg) have increased
mortality after liver transplantation
• HPS often resolves completely
after transplant
Pathophysiology of ESLD
• Pulmonary changes
– Hepatic hydrothorax
• Seen in 5-10% of ESLD patients
• Pleural effusion from transfer of ascitic fluid
through diaphragmatic defects
• Treated by sodium restriction, diuretics
and/or thoracentesis
Pathophysiology of ESLD
• Pulmonary changes
– Pulmonary hypertension
• Seen in <5% of ESLD patients
• Defined as mean pulmonary artery pressure (MPAP)
>25mmHg and increased pulmonary vascular resistance
– Patients with MPAP >35mmHg have increased
perioperative morbidity/mortality
– Patients with MPAP >50mmHg, at VAPHS, are not transplant
candidates secondary to greatly increased mortality
• Etiology not well understood
Pulmonary Hypertension
• Avoid physiologic conditions that increase
pulmonary vascular resistance, as acute
right-sided heart failure can result
– Hypoxemia
– Hypercapnia
– Acidosis
• Important to remember during monitored
anesthesia care (MAC) cases
Pathophysiology of ESLD
• Renal changes
– Impaired free water and sodium excretion
– Decreased renal perfusion and glomerular
filtration rate (GFR)
– Vasodilation, which effectively reduces plasma
volume, leads to sympathetic nervous system
activation of the renin-angiotension-aldosterone
pathway, resulting in enhanced sodium and free
water resorption
Pathophysiology of ESLD
• Renal changes lead to development of
– Edema
– Ascites
• Long term decrease in renal perfusion and
GFR can lead to hepatorenal syndrome (HRS)
– HRS occurs in up to 10% of patients with ESLD
– Functionally HRS is a pre-renal phenomenon
whose hallmark is intense renal
vasoconstriction
Hepatorenal Syndrome (HRS)
• Type I
– Progressive oliguria with rapidly rising creatinine
– Often follows an episode of spontaneous
bacterial peritonitis (SBP)
– Poor outcome – median survival < 1 month
without intervention
– Treatment with albumin, octreotide, and
midodrine has shown some promise
Hepatorenal Syndrome (HRS)
• Type II
– Usually seen in patients with refractory ascites
– Renal impairment is usually more mild than type I
– Clinical course is far less progressive than type I
Pathophysiology of ESLD
• Ascites
– Common complication of ESLD; in fact, nearly
50% of patients develop ascites within 10
years of initial diagnosis
– Significant associated mortality – nearly 50% of
patients die within 3 years of onset of ascites
– Etiology complex, multifactorial and not
completely understood
• Portal hypertension
• Sodium, water retention
Pathophysiology of ESLD
• Ascites
– Treatment
• Sodium restriction and diuretics (spironolactone)
• Refractory cases treated with repeated large-volume
paracentesis and volume expanders, usually albumin
• Transjugular intrahepatic portosystemic shunt (TIPS)
also can be used for refractory ascites, but it has not
been shown to improve survival compared to repeat
paracentesis
Anesthesia and ESLD
• Preoperative preparation should focus on
optimizing liver-related pathology (if possible)
– Volume status
– Coagulation – parenteral vitamin K if INR elevated
– Renal function
– Electrolyte imbalance
– Nutritional status
Anesthesia and ESLD
• Medications should be scrutinized in the
preoperative period, as there are a large
number that can cause or worsen
underlying hepatic dysfunction
– Acetaminophen
– Isoniazid
– Methyldopa
– Phenytoin
– Indomethacin
Anesthesia and ESLD
• Administration of anesthesia decreases liver
blood flow via changes in hepatic perfusion
pressure and/or splanchnic vascular resistance
• Physiologic reserve is decreased patients
with ESLD
• Perioperative morbidity and mortality in
patients undergoing all but minor
procedures is increased
Child-Pugh Classification System
Child-Pugh Class and Mortality
• Thirty day mortality in patients undergoing
either cholecystectomy, hernia repair, GI or
miscellaneous surgery; 25% were emergencies
– Class A – 10%
– Class B – 30%
– Class C – 80%
• Highest mortality in GI and emergent
procedures
Child-Pugh Class and Mortality
• Three month mortality for patients
hospitalized with liver complications,
but not undergoing surgery
– Class A – 4%
– Class B – 14%
– Class C – 50%
Model for End-Stage Liver Disease
(MELD) Score
• Originally developed to predict survival in
patients with portal hypertension undergoing
elective TIPS procedures
• Found to be an accurate predictor of survival
in patients with a variety of liver diseases
• Adopted in 2002 as the rank list criteria for
liver transplantation by the United Network of
Organ Sharing (UNOS), replacing
Child-Pugh
Model for End-Stage Liver Disease
(MELD) Score
• Resulted in an almost 15% reduction in
mortality on the waiting list
• Median waiting time also decreased, about
35%, from 656 to 416 days
• While it accurately estimates mortality on the
waiting list, MELD does not correlate well with
mortality following liver transplantation
MELD Score
Anesthesia and ESLD
• Perioperative mortality calculator
– http://www.mayoclinic.org/medicalprofessionals/model-end-stage-liver-disease/postoperative-mortality-risk-patients-cirrhosis
– Input patient age, ASA physical status, bilirubin,
creatinine, INR and cirrhosis etiology
(alcoholic/cholestatic vs viral/other)
– Calculates mortality at 7, 30 and 90 days,
as well as 1 and 5 years
Anesthesia and ESLD
• 52 year male presenting for R total knee
arthroplasty
– PMHx HTN, DM, hep C, CKD, COPD, GERD, PTSD
– Lab data – HgB 11, platelets 95K, K 4, BUN/Cr
20/1.4, total bili 1.5, PT/INR 15.7/1.3
• Does this patient have a significant degree
of morbidity/mortality in the perioperative
period?
Anesthesia and ESLD
• YES!!!
– 7 day mortality
– 30 day mortality
– 90 day mortality
– 1 year mortality
– 5 year mortality
2.719%
10.696%
16.698%
29.079%
61.382%
Anesthesia and ESLD
• Pharmacokinetic and pharmacodynamic
considerations
– Multiple aspects possibly affected
•
•
•
•
Hepatic metabolism
Renal metabolism
Volume of distribution
Protein binding
– All medications should be titrated to effect
• “You can always give more”
Anesthesia and ESLD
• Intraoperative management
– Anesthetic technique
• No one medication, technique or approach has
proven superior in patients with ESLD
• MAC and regional are appropriate, but need to be
considered on a case-by-case basis
– All medications should be titrated to effect
Intraoperative Management
• Overall hepatic blood flow is decreased
due to portal hypertension
– Hepatic oxygenation, therefore, is more
dependent on hepatic artery blood flow
than normal
– Volatile anesthetics blunt the ability of the
hepatic artery to vasodilate in the face of
decreased portal vein blood flow
Intraoperative Management
• Overall hepatic blood flow is decreased
due to portal hypertension
– Any decrease in systemic blood pressure,
for example from volatile anesthetic-induced
peripheral vasodilation, can decrease hepatic
artery blood flow
• Probably best to avoid delivering high
concentrations of volatile agents to
patients with ESLD
Intraoperative Management
• Monitoring and vascular access
– Standard American Society of Anesthesiology
(ASA) monitors
– Additional invasive monitors as dictated by
• Degree of liver disease
• Presence/absence of other underlying disease
• Nature of surgical procedure
Intraoperative Management
• Monitoring and vascular access
– Other considerations
•
•
•
•
Urine output
“Gentle” esophageal manipulation – varices
Bispectral index (BIS)
Real-time coagulation assessment –
thromboelastography (TEG)
– Vascular access
• Large bore catheter(s) recommended
Intraoperative Management
• Induction of general anesthesia (GA)
– Rapid sequence vs routine induction
– Does the presence of ascites = full stomach?
– Succinylcholine may have a prolonged
duration of action due to decreased
plasma cholinesterase activity
– Theoretically may need larger initial dose of
non-depolarizing muscle relaxants due to
increased volume of distribution, especially in
those patients with significant ascites
Intraoperative Management
• Maintenance of GA
– The golden rule – maintain homeostasis
•
•
•
•
•
Avoid hypotension
Avoid low cardiac output
Avoid bradycardia
Avoid myocardial depression
Avoid peripheral vasodilation
– Remember, pressure = flow X resistance
(BP = CO X SVR)
Intraoperative Management
• Maintenance of GA
– Halothane hepatitis
•
•
•
•
Diagnosis of exclusion
Autoimmune vs hepato-toxic metabolites
~1:35,000 incidence of fatal hepatic necrosis
Risk factors
–
–
–
–
Middle age
Obesity
Female gender
Repeated exposure, especially within 28 days
Intraoperative Management
• Maintenance of GA
– Muscle relaxants
• Metabolism of both rocuronium and vecuronium
is 60-90% dependent on hepatic degradation
and biliary excretion
• Pancuronium relies mostly on renal excretion (80%)
but about 20% of metabolism occurs via the liver
• Cisatracurium, by nature of its organ-independent
clearance via Hofmann degradation, is ideal to use
in patients with ESLD
Intraoperative Management
• Maintenance of GA
– Fluid therapy
• No prospective data exist showing a benefit
to crystalloid vs colloid
• Maintenance of adequate filling pressure is more
important than the choice of fluid
– Blood transfusion
• Communication with blood bank is crucial
• RBCs, FFP, platelets, cryoprecipitate
Intraoperative Management
• Vasoactive medications
– ESLD patients typically are in a hyperdynamic,
vasodilated state
– Anesthetic-induced increases in peripheral
vasodilation can lead to profound hypotension
– Administration of vasoconstricting agents –
phenylephrine, norepinephrine, vasopressin –
is common and dosing is often higher than
normally required
Intraoperative Management
• Blood transfusion
– Lab turnover time may render traditional
coagulation testing (i.e. PT/INR, PTT, platelet
count) irrelevant during high-volume blood loss
cases in ESLD patients
– Thromboelastography (TEG)
• Allows real-time assessment of all aspects of
the coagulation cascade
• Used in cardiac surgery, trauma and liver
transplantation
Thromboelastography (TEG)
Thromboelastography (TEG)
• Components
– R, reaction time: time until initial clot formation
– K, clot formation time: period after R to achieve
clot width of 20mm
– α, alpha angle: measures speed of clot formation
– MA, maximum amplitude: measure of the
strength of the fully formed clot
– A60/MA ratio: compares maximum clot
size to that 60 minutes later
Thromboelastography (TEG)
• Treatment decisions
– Prolongation of R and/or K, or a decrease in α,
treated with fresh frozen plasma (FFP)
– Decrease in MA treated with platelets
– A60/MA < 0.85 indicates fibrinolysis and is
treated with epsilon-aminocaproic acid
• Use of TEG during liver transplantation has
been shown to reduce the amounts of RBCs
and FFP transfused
Orthotopic Liver Transplantation
(OLTx)
• VA Pittsburgh Healthcare System experience
– Started doing liver transplants in the mid-1980s
– Dedicated VA transplant surgeon since Jan 2004
– One year survival 84.6%; national average 90.2%
• Latest data per UNOS
• Period ending December 31, 2012
OLTx at VAPHS
•
•
•
•
Cases are always emergent
All adult patients
Frequently occur after hours or on weekends
Separate, dedicated call team
– Anesthesiologist
– CRNA
– Anesthesia technician
OLTx at VAPHS
Common ESLD etiologies
Uncommon etiologies
• Hepatitis C
• Alcoholic cirrhosis
• Hepatocellular carcinoma
(HCC)
• Some combination of the
three
• Primary sclerosing
cholangitis
• Primary biliary cirrhosis
• Non-alcoholic steatohepatitis (NASH)
• Autoimmune hepatitis
OLTx at VAPHS
• Preoperative considerations
– All patients undergoing multi-disciplinary
evaluation, including surgery, anesthesiology
and psychiatry consultation
– Workup includes a full battery of lab tests,
ECG, CXR and PFTs
Preoperative Considerations
• Cardiopulmonary workup includes
– Stress testing
• Low threshold for cardiac catheterization
• Case-by-case decision, but generally any patient with
more than mild CAD is not a surgical candidate
– Transthoracic echocardiography
• Pulmonary artery pressure (PAP) estimation
• Mean PAP >35mmHg associated with increased
perioperative morbidity/mortality;
patients with MPAP >50mmHg are not
surgical candidates
OLTx at VAPHS
• Intraoperative considerations
– Standard ASA monitors plus
• BIS
• Arterial line – right femoral
• Large-bore iv access – “double stick”
– Right internal jugular (RIJ) 9Fr double-lumen introducer
with pulmonary artery catheter (PAC)
– RIJ veno-venobypass (VVB) cannula, 18Fr
– Ultrasound guidance
• Pacing/defibrillator pads
OLTx at VAPHS
• Intraoperative considerations
– Emergency case – “full stomach” management
– Anesthetic maintenance
• Usually a balanced technique
• My preference is more toward a cardiac anesthetic
– High dose benzodiazepine/opioid dosing
– Low, steady state dose of volatile anesthetic
– Muscle relaxant – dealer’s choice
Intraoperative Considerations
• Coagulation management
– Arterial blood gas (ABG) and TEG done hourly
– PT/INR/PTT/platelets done every 2 or 3 hours
– Reperfusion resets the lab timeline
– Additional labs as needed
Intraoperative Considerations
• Rapid infusion system (RIS)
– In OR and primed for every case
– Decision to use made on a case-by-case basis
• Cell-saver blood salvage system
– Set up for every case
– Not employed until after reperfusion in VAPHS
patients with hepatocellular CA
Intraoperative Considerations
• OLTx procedure can be broken down
into 3 phases
– Pre-anhepatic
– Anhepatic
– Post-anhepatic or neohepatic
• Average operative time at VAPHS ~8 hours
Pre-anhepatic Phase
• Lasts from skin incision to the point when the
native liver is freed to its vascular pedicle
• Native liver is mobilized
– Hilum located
– Hepatic artery (HA) ligated
– Bile duct transected
– Infra- and supra-hepatic inferior vena cava (IVC)
and portal vein (PV) encircled
Pre-anhepatic Phase
• May involve veno-venobypass (VVB)
– Venous outflow via 2 cannulas – L common iliac
vein and portal vein
– Venous return via 1 cannula – R internal jugular
(may use the L axillary vein via cutdown)
• VVB complications include
– Hypothermia
– Air or thromboembolism
– Brachial plexus and/or vessel trauma
Anhepatic Phase
• Lasts from clamping of the infra- and suprahepatic IVC, PV and hepatic artery and ends
when the IVC and PV anastomoses are
complete
• Usually see decreased cardiac output/index
from decreased venous return
• Use of VVB can lead to profound hypothermia,
especially if a heat exchanger is not used
Anhepatic Phase
• Portal hypertension does NOT protect
against hemodynamic instability
• Anastomotic order
– Supra-hepatic IVC
– Infra-hepatic IVC
– Portal vein
– Hepatic artery
Anhepatic Phase
• Reperfusion
– Occurs when PV (inflow) and IVC (outflow)
anastomoses are complete
– Often associated with hemodynamic instability
• Bradycardia, asystole
• Hypotension
• Hyperkalemia, despite donor organ flush, may result
from preservation solution with high K+ concentration
• Air or thromboembolism
• Pulmonary hypertension, often with acute
right heart failure, is rare but may occur
Anhepatic Phase
• Reperfusion
– Post-reperfusion syndrome
• Decrease in mean arterial pressure of at least 30% for
at least 1 minute within 5 minutes of reperfusion
• Usually see bradycardia, high filling pressures and
peripheral vasodilation (↓ SVR)
• Washout of “evil humors” from the donor organ –
kinins, cytokines, free radicals
• Usually responds to vasoconstrictors –
phenylephrine, norepi or vasopressin
Anhepatic Phase
• Reperfusion
– Preparation should include
• Ventilation with 100% FiO2
• Priming/filling of the RIS, if in use
• Vasoactive meds/infusions in line
– Epinephrine
– Calcium
– Phenylephrine, norepi, vasopressin
• Pacer connected, turned on
Post-anhepatic (Neohepatic) Phase
• Begins with PV and HA unclamping
• Biliary drainage is reconstructed
– End-to-end anastomosis, often with T-tube
– Roux-en-Y choledochojejunostomy
• Fibrinolysis may occur – epsilon-aminocaproic
acid 500mg-1g iv if present on TEG
• Unclamping order – PV, infra-hepatic IVC,
supra-hepatic IVC, HA and lastly
the bile duct
OLTx at VAPHS
• End of case management
– VVB cannula removal – needs purse-string suture
– ETT, arterial line and introducer/PAC stay in
– Transport with full monitors and 100% FiO2 via
ambu to intensive care
• Post-op day #1, etc
– Extubation and invasive monitor removal
depend on graft function, co-existing
disease & patient status preoperatively
Anesthetic Management of Patients
after OLTx
• Following successful OLTx, liver synthetic
function and metabolic activity return
to normal
– Lab values normalize
– Normal hepatic drug clearance
• Circulation no longer hyperdynamic
• Oxygenation generally improves,
although some anatomic V-Q
mismatch may persist
Anesthesia after OLTx
• No routine lab work (PT/INR/platelets/LFTs)
needed for patients with normally
functioning grafts
• Problems arise from adverse effects
(anemia, thrombocytopenia) of and/or
drug interactions with chronic
immunosuppressive therapy
Anesthesia after OLTx
• Short-term complications
– Technical considerations
• Hepatic artery thrombosis (HAT)
• Portal vein thrombosis – less common
• Bile duct leak
– Primary graft non-function
– Infection
• In these situations refer to previous slides
as patients will physiologically once
again have ESLD
Anesthesia after OLTx
• Long-term complications
– Chronic kidney disease occurs more
frequently in patients with
• Diabetes
• Hepatitis C
– Diabetes occurs more commonly in patients
with hep C
– Infection
Anesthesia after OLTx
• Long-term complications
– Coronary artery disease
• Risk factors – older age at transplant, male sex,
post-transplant diabetes or hypertension
– Infection
– These long-term problems are managed in the
usual fashion, irrespective of the OLTx history
References
• Barash, Paul G, et al. Clinical Anesthesia. 6th ed.
Philadelphia: Lippincott Williams & Wilkins, 2009.
• Miller, Ronald D, et al. Miller’s Anesthesia. 7th
ed. Philadelphia: Churchill Livingstone, 2010.
• Jaffe, Richard A, et al. Anesthesiologist’s Manual
of Surgical Procedures. 4th ed. Philadelphia:
Lippincott Williams & Wilkins, 2009.
• Winter, Peter M., and Yoo Goo Kang. Hepatic
Transplantation. New York: Praeger, 1986.
References
• Murray JF, et al. Circulatory changes in chronic liver
disease. Am J Med 1958; 24: 358.
• Sharara AI and Rockey DC. Gastroesophageal variceal
hemorrhage. N Engl J Med 2001; 345: 669.
• Salerno F, et al. Transjugular intrahepatic
portosystemic shunt for refractory ascites: a metaanalysis of individual patient data. Gastroenterology
2007; 133: 825.
• Rodriguez-Roisin R and Krowka MJ. Hepatopulmonary
syndrome – a liver-induced lung vascular disorder. N
Engl J Med 2008; 358: 2378-87.
References
• Follo A, et al. Renal impairment after spontaneous
bacterial peritonitis in cirrhosis: Incidence, clinical
course, predictive factors and prognosis. Hepatology
1994; 20: 1495.
• Wadei HM, et al. Hepatorenal syndrome:
pathophysiology and management. Clin J Am Soc
Nephrol 2006; 1: 1066.
• Kujovich JL. Hemostatic defects in end stage liver
disease. Crit Care Clin 2005; 21: 563-87.
• Senzolo M, et al. New insights into the coagulopathy of
liver disease and liver transplantation. World J
Gastroenterol 2006; 12(48): 7725-36.
References
• Tripodi A and Mannucci PM. The coagulopathy of
chronic liver disease. N Engl J Med 2011; 365: 147-56.
• Pugh RNH, et al. Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg 1973; 60: 646.
• Kamath PS, et al. A model to predict survival in
patients with end-stage liver disease. Hepatology 2001;
33: 464.
• Kamath PS and Kim WR. A model for end-stage liver
disease (MELD). Hepatology 2007; 45: 797-805.
References
• Mansour A, et al. Abdominal operations in patients with
cirrhosis: still a major surgical challenge. Surgery 1997;
122: 730.
• Kang YG, et al. Epsilon-aminocaproic acid for treatment of
fibrinolysis during liver transplantation. Anesthesiology
1987; 66: 766-773.
• Albeldawi M, et al. Cumulative risk of cardiovascular
events after orthotopic liver transplantation. Liver
Transplant 2012; 18: 370-5.
• McGuire BM, et al. Long-term management of the liver
transplant patient: recommendations for the primary care
doctor. Am J Transplant 2009; 9: 1988-2003.