Clinical Epidemiology Boot Camp Systematic Reviews
advertisement

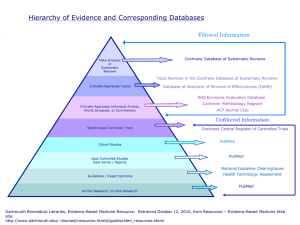
Clinical Epidemiology Boot Camp: Systematic Reviews Selina Liu MD MSc FRCPC Research Fellow Resident Research Career Development Program September 19, 2012 Outline Introduction – Evidence-Based Medicine (EBM) Levels of evidence To discuss the definition of a systematic review vs. traditional/narrative reviews The process of conducting a systematic review Strengths & limitations of systematic reviews To describe how to critically appraise a systematic review Example of a systematic review Evidence-Based Medicine What is Evidence-Based Medicine? “…the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients” “ It’s about integrating individual clinical expertise and the best external evidence” philosophical origins – date back to mid-19th century Paris (or possibly earlier) Sackett DL et al. BMJ. 1996;312(7023):71-2 Evidence-Based Medicine Five Steps of Evidence-Based Medicine 1. Asking Focused Questions 2. Finding the Evidence Testing evidence for validity, clinical relevance, and applicability 4. Making a Decision Systematic retrieval of the best evidence available 3. Critical Appraisal Translation of uncertainty to an answerable question Application of results in practice 5. Evaluating Performance Auditing evidence-based decisions Oxford Centre for Evidence-Based Medicine (CEBM) www.cebm.net Evidence-Based Medicine Why Evidence-Based Medicine? clinical decision making is complex! Mulrow CD, Cook DJ, Davidoff F. Ann Intern Med. 1997;126(5):389-91 Evidence-Based Medicine How do we practice Evidence-Based Medicine? Can be difficult: “information overload” difficult for clinicians to “keep up” with all of the latest evidence often there are multiple studies examining the same or similar questions may be of variable quality, generalizability estimated time required for reading (general medicine): 19 articles per day, 365 days per year Davidoff F et al. BMJ. 1995;310(6987):1085-6 Evidence-Based Medicine Weighing the evidence - “Levels of Evidence” OCEBM Levels of Evidence Working Group. “The Oxford 2011 Levels of Evidence”, Oxford Centre for Evidence-Based Medicine. www.cebm.net/index.aspx?o=5653 Evidence-Based Medicine of RCTs Systematic reviews of cohort studies Systematic Reviews What is a Systematic Review? the application of strategies that limit bias in the assembly, critical appraisal, and synthesis of all relevant studies on a specific topic use rigorous, standardized methods for selecting & assessing articles Oxford Centre for Evidence-Based Medicine www.cebm.net/?o=1116 OR a report that summarizes all evidence that can be drawn from research (or other sources), that is relevant to a specific clinical question Systematic Reviews Systematic Reviews vs. Traditional Review Articles traditional review articles written by senior expert in the field, summarizes evidence and recommendations usually address broad areas/questions (i.e. “management of T2DM”) often lack structure may include personal experience/anecdotal evidence Fletcher RH & Fletcher SW 2005. Clinical Epidemiology: The Essentials Systematic Reviews Systematic Review vs. Traditional/Narrative Review Cook DJ, Mulrow CD, Haynes RB. Ann Intern Med. 1997;126(5):376-380 Systematic Reviews Guyatt G et al. 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice (2nd Edition) Systematic Reviews Process of Conducting a Systematic Review 1. Define the question 2. Conduct literature search 3. Apply inclusion and exclusion criteria 4. Create data abstraction 5. Conduct analysis Guyatt G et al. 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice (2nd Edition) Systematic Reviews - Process 1. Define the Question single, focused question i.e. What is the effect of cinnamon on glycemic control in diabetes? specify inclusion and exclusion criteria Population, Intervention or Exposure, Outcome, Methodology For the systematic review to be useful: strong studies of the question should be available, but their results should not be so much in agreement that the question is already answered! there should not be so few studies of the question that each individual study could be fully critiqued directly Systematic Reviews - Process 2. Conduct literature search need to ensure that all of the appropriate studies are included decide on information sources NOT just a biased sample of studies i.e. MEDLINE, recent reviews, textbooks, experts in the field, articles cited by references already found by other approaches, databases of articles, clinical trial registries etc. identify titles and abstracts Systematic Reviews - Process 3. Apply inclusion and exclusion criteria Apply inclusion and exclusion criteria to titles and abstracts obtain full articles for eligible titles and abstracts Apply inclusion and exclusion criteria to full articles Select final eligible articles Assess agreement on study selection of the initial titles and abstracts retrieved, usually only a small proportion of articles are selected Systematic Reviews - Process 4. Create data abstraction Assess methodologic quality of each article Assess agreement on validity assessment Data abstraction Participants Interventions and Comparison Interventions Study Design Results Systematic Reviews - Process 5. Conduct analysis Summarize data If appropriate: meta-analysis – statistical technique to combine quantitative data Describe results – often graphically usually combine studies vs. combine patients Forest Plot – shows point estimate and confidence interval (for RCTs, observational studies) Summary Receiver-Operator Curves (for studies of diagnostic tests) Explore heterogeneity, conduct subgroup analysis (if appropriate) Explore possibility of publication bias (and other biases) Systematic Reviews - Process How to decide if appropriate to perform a meta-analysis? Two general approaches: 1. statistical test for homogeneity BUT – even if fail to reject H0 (i.e. no evidence of a statistically significant difference between studies), usually have high risk of false-negative (saying studies are homogeneous when they really are not) Limited power - meta-analyses are usually of few number of studies, - affected also by number of patients/study, distribution of patients among studies 2. informed judgement Systematic Reviews - Process Meta-analysis – mathematical models: Fixed-Effect Model Assumes that studies are of exactly the same question, so results differ only by chance Confidence intervals calculated may imply more precision (i.e. are narrower) than in reality (since studies usually differ somewhat) Random-Effects Model Assumes that the studies address somewhat different questions, but that they form a closely related family of studies of a similar question Studies taken to be a random sample of all studies bearing on the question Produces WIDER confidence intervals (more “realistic”) Fletcher RH & Fletcher SW. 2005. Clinical Epidemiology: the Essentials (4 th Edition) Systematic Reviews – Forest Plot Impact of Treatment on Mortality Study name Kelly, 1964 Hedrin, 1980 Leigh, 1962 Novak, 1992 Saint, 1998 Pilbean, 1936 Day, 1960 Kelly, 1966 Singh, 2000 Stewart, 1994 Statistics for each study Odds ratio Lower limit 0.590 0.464 0.394 0.490 1.250 0.129 0.313 0.429 0.718 0.143 0.328 0.096 0.201 0.076 0.088 0.479 0.027 0.054 0.070 0.237 0.082 0.233 Odds ratio and 95% CI Upper limit 3.634 1.074 2.055 2.737 3.261 0.605 1.805 2.620 2.179 0.250 0.462 0.01 0.1 Favours Tx Meta Analysis 1 10 Favours Pbo 100 Systematic Reviews - Bias Several types of bias: publication bias language bias i.e. if only English-language articles are selected size bias published studies may be systematically different than unpublished studies (“positive” studies vs. “negative” studies?) large studies that result in several publications may be more readily noticed than smaller studies bias related to funding? industry-sponsored studies How to detect publication bias? Funnel plots – plot effect vs. study size/precision symmetrical, peaked distribution (inverted funnel) Guyatt G et al. 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice (2nd Edition) How to detect publication bias? Funnel plots asymmetrical distribution Guyatt G et al. 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice (2nd Edition) Systematic Reviews - Strengths provide an efficient way to become familiar with the best available research evidence for a focused clinical question can establish whether results are consistent, generalizable across populations/settings, treatment variations, and whether findings vary by certain subgroups can extend the available literature (if the review team has obtained unpublished information from the primary authors) meta-analyses – may provide a more precise estimate of the underlying “true effect” than any individual study Garg AX, Hackam D, Tonelli M. 2008. Clin J Am Soc Nephrol. 3(1):253-60. Systematic Reviews - Limitations summarized results are limited by the quality of the primary studies garbage out” results dependent on selection of included articles “garbage in quality threshold, publication bias, language bias etc. meta-analyses - may be inappropriate to mathematically combine primary study results if the primary studies differ in design, quality, population, intervention etc. subjectivity involved in deciding whether to pool or not subjectivity in interpretation of summarized results (“overinterpretation) Garg AX, Hackam D, Tonelli M. 2008. Clin J Am Soc Nephrol. 3(1):253-60. Systematic Reviews – Critical Appraisal Oxman AD, Cook DJ, Guyatt GH, Evidence-Based Medicine Working Group. JAMA. 1994;272(17):1367-71 Tonelli M, Hackam D, Garg AX. Methods Mol Biol. 2009;473:217-33 Critical Appraisal – Tools Oxford Centre for Evidence-Based Medicine http://www.cebm.net/index.aspx?o=1157 Critical Appraisal – Tools Oxford Centre for Evidence-Based Medicine http://www.cebm.net/index.aspx?o=1157 Critical Appraisal – Tools AMSTAR – 2007 Assessment of Multiple SysTemAtic Reviews Shea BJ, Grimshaw JM, Wells GA et al. 11 item tool developed via exploratory factor analysis of a 37-item assessment tool Shea BJ, Grimshaw JM, Wells GA et al. BMC Med Res Methodol. 2007;7:10 Critical Appraisal - Tools Shea BJ, Grimshaw JM, Wells GA et al. BMC Med Res Methodol. 2007;7:10 Reporting Systematic Reviews - Tools The PRISMA Statement – 2009 Preferred Reporting Items for Systematic reviews and MetaAnalyses Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group PLoS Medicine Annals of Internal Medicine BMJ Journal of Clinical Epidemiology Open Medicine International Journal of Surgery The PRISMA Statement - 2009 Aim – to help authors improve the reporting of systematic reviews and meta-analyses **NOT intended to be a quality assessment tool 27 item checklist four-phase flow diagram update and expansion of prior QUOROM statement QUality Of Reporting Of Meta-analyses - 1996 focused on reporting of meta-analyses of randomized controlled trials Table 1. Checklist of items to include when reporting a systematic review or meta-analysis. Moher D, Liberati A, Tetzlaff J, Altman DG, et al. (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed.1000097 Figure 1. Flow of information through the different phases of a systematic review. Moher D, Liberati A, Tetzlaff J, Altman DG, et al. (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed.1000097 Example of a Systematic Review Cochrane Database of Systematic Reviews 2012, Issue 9:CD007170 Cinnamon for Diabetes Mellitus Objective – to evaluate the effects of cinnamon in patients with diabetes mellitus Selection criteria – all RCTs comparing the effects of orally administered monopreparations of cinnamon (Cinnamomum spp.) to placebo, active medication or no treatment in persons with either type 1 or type 2 diabetes mellitus Primary outcomes – fasting blood glucose, post-prandial glucose, adverse events Secondary outcomes – HbA1c, serum insulin, HOMA-IR, HRQoL, morbidity, costs Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Risk of Bias Summary Review authors’ judgements about each “risk of bias” item for each included study Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Risk of Bias Graph Review authors’ judgements about each “risk of bias” item presented as percentages across all included studies Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Results – Cinnamon vs. Placebo Primary outcome – fasting blood glucose Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Results – Cinnamon vs. Placebo Primary outcome – adverse events Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Results – Cinnamon vs. Placebo Secondary outcome – HbA1c Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Results Other primary outcomes: Post-prandial glucose – 1trial Other secondary outcomes: Serum insulin – 2 trials HOMA-IR – 2 trials HRQoL, morbidity, costs – no trials Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Conclusions Insufficient evidence to support the use of cinnamon for type 1 or type 2 diabetes Further trials required: To address methodologic issues in current studies i.e. allocation concealment, blinding To include other important endpoints HRQOL, diabetes complications, cost Leach MJ & Kumar S. Cochrane Database of Syst Rev 2012, Issue 9:CD007170 Useful Resources Guyatt G, Rennie D, Meade MO, Cook DJ. 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice (2nd Edition). New York NY, McGraw-Hill Oxford Centre for Evidence-Based Medicine (CEBM) available online via Western Libraries www.cebm.net Cochrane Database of Systematic Reviews www.thecochranelibrary.com References Sackett DL, Rosenberg WMC, Muir Gray JA, Haynes RB, Richardson WS. BMJ. 1996;312(7023):71-2 Mulrow CD, Cook DJ, Davidoff F. Ann Intern Med. 1997;126(5):389-91 Davidoff F, Haynes B, Sackett D, Smith R. BMJ. 1995;310(6987):1085-6 Oxford Centre for Evidence-Based Medicine (CEBM) www.cebm.net OCEBM Levels of Evidence Working Group. “The Oxford 2011 Levels of Evidence”, Oxford Centre for Evidence-Based Medicine. www.cebm.net/index.aspx?o=5653 Fletcher RH & Fletcher SW. 2005. Clinical Epidemiology: the Essentials (4th Edition). Baltimore MD, Lippincott Williams & Wilkins Guyatt G, Rennie D, Meade MO, Cook DJ. 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice (2nd Edition). New York NY, McGraw-Hill Cook DJ, Mulrow CD, Haynes RB. Ann Intern Med. 1997;126(5):376-380 Garg AX, Hackam D, Tonelli M. 2008. Clin J Am Soc Nephrol. 3(1):253-60. Oxman AD, Cook DJ, Guyatt GH, Evidence-Based Medicine Working Group. JAMA. 1994;272(17):1367-71 Tonelli M, Hackam D, Garg AX. Methods Mol Biol. 2009;473:217-33 Cochrane Database of Systematic Reviews www.thecochranelibrary.com