Managing and Coordinating the Semi-annual Site Visit
advertisement
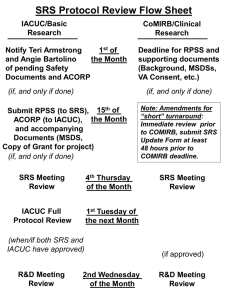
Managing and Coordinating the Semiannual Site Visit Process Where do I start? What do I need to follow? Alphabet Soup of Guidance Documents, Regulatory Bodies, & Accreditation Association Materials References have been paraphrased as much as possible While site visits typically are part of the programmatic review/report, we’ll focus on the site visit details Guide for the Care & Use of Laboratory Animals – 8th edition …facilities inspection should occur at least annually or more often as required (e.g., by the Animal Welfare Act and PHS Policy)… PHS Policy What’s required? inspect at least once every six months all of the institution’s animal facilities (including satellite facilities) using the Guide as a basis for evaluation animal facility - all buildings, rooms, areas, enclosures, or vehicles, including satellite facilities, used for animal satellite facility - any containment outside of a core facility/centrally designated or managed area housing animals Footnote 8 - 8 The IACUC may, at its discretion, determine the best means of conducting an evaluation of the institution’s confinement, transport, maintenance, breeding, or experiments inclusive of surgical manipulation. >24 hours. programs and facilities. They may invite ad hoc consultants to assist in conducting the evaluation, but they remain responsible for the evaluation and report. prepare semiannual report, include site visit deficiencies distinguish significant from minor deficiencies significant deficiency - in the judgment of the IACUC and the IO, is or may be a threat to the health or safety of the animals. reasonable and specific plan and schedule for correcting each deficiency PHS Assurance (& Renewals) If your program is not AAALAC International accredited, the most recent semiannual report of the IACUC evaluation shall be submitted to OLAW with the Assurance. PHS - OLAW FAQs E1. Should the IACUC inspect laboratories or other sites where investigators use animals? Institutions are responsible for oversight of all animal-related activities regardless of how long or where the activity occurs. Satellite facilities (housed for more than 24 hours outside a core/centrally managed area) and areas where any form of surgical manipulations (minor, major, survival, nonsurvival) are performed must be inspected at least once every six months by the IACUC. Institutions have discretion with regard to how they oversee areas used for routine weighing, dosing, immunization, or imaging, but should monitor such areas on a random or fixed schedule to effectively oversee activities at the institution. [A1, A7] Reference A1. (ILAR News 1991; 33(4)) …must the IACUC inspect every laboratory or other site where investigators use animals? PHS Policy allows institutions some discretion regarding specific methods to assure compliance the institution is clearly responsible for what happens to animals in investigators' laboratories the degree, method, and frequency of IACUC oversight depends a great deal on the nature of the activity activities, such as routine dosing, weighing, or immunization of animals in laboratories, may be monitored using other methods such as random evaluation PHS - OLAW FAQs E4. Is the IACUC required to inspect field study sites? Semiannual IACUC inspections of field study sites are not required and in many circumstances are impractical. IACUCs should be apprised of the circumstances under which studies are conducted so that they can consider risks to personnel and impact on study subjects. This may be partially accomplished by written descriptions, photographs, or videos that document specified aspects of the study site. The IACUC should also ensure that appropriate permits are in place. AWA & Regulations AWA: 2143(b)(4-5) and AWR: 2.31(c)(2-3) (2) Inspect, at least once every six months, all of the research facility's animal facilities, including animal study areas (area outside of the central/core facility where animals are housed for more than 12 hours) That animal areas containing free-living wild animals in their natural habitat need not be included in such inspection (3) Prepare reports of the evaluations, and submit the reports to the Institutional Official of the research facility the IACUC may determine the best means of conducting evaluations of the research facility's programs/facilities no Committee member wishing to participate may be excluded the IACUC may use subcommittees (at least two Committee members) and may invite ad hoc consultants to assist distinguish significant from minor deficiencies (significant deficiency - in the judgment of the IACUC and the IO, is or may be a threat to the health or safety of the animals) a reasonable and specific plan and schedule with dates for correcting each deficiency; **any failure to adhere to the plan and schedule that results in a significant deficiency remaining uncorrected shall be reported in writing within 15 business days by the IACUC, through the Institutional Official, to APHIS and any Federal agency funding that activity** AAALAC International Applies only to accredited sites/animals – FAQ C2: AAALAC International expects program reviews and facility inspections by the IACUC (or comparable oversight body) be conducted at a frequency and intensity that ensure prompt identification of program issues, with rapid correction of identified deficiencies. Looks for evidence of a highly engaged Committee that: conducts thorough evaluations of the program and facilities, ensures corrective measures are taken in a timely manner, and that the program and facilities are adequately supporting the research, testing and teaching objectives of the institution. Program reviews and facilities inspections can be an effective component of overall monitoring and oversight. Committees are encouraged to carefully consider the frequency of their evaluations in order to ensure quality animal care and science, which may be more frequently than required by other regulations/guidelines. AAALAC Site Visits OLAW FAQ E3 May the IACUC use an AAALAC International site visit as its semiannual evaluation? NOT-OD-00-007. AAALAC assessment programs or pre-assessment preparation activities may be utilized as ad hoc consultants to meet the requirements for an IACUC semiannual program evaluation. To utilize one of these AAALAC- related activities as a semiannual evaluation, the IACUC must ensure that the following provisions of the PHS Policy and USDA animal welfare regulations, as applicable, are met: …the program review must comply with PHS Policy…including a plan and schedule for correcting each deficiency… For institutions covered by USDA animal welfare regulations: at least two IACUC members must participate in the evaluation no IACUC member wishing to participate may be excluded The Animal Care unit of the USDA Animal and Plant Health Inspection Service and AAALAC have reviewed and concur with the guidance provided in this notice. Effective Communication Determining the schedule of the visits by location? by facility type? Lumpers & splitters condense/intense spread out/keep track of timing Rounding up IACUC member participation Use of Primary Members Use of Alternates (when Primary member is unavailable) Use of AV and their designee(s) as members of the IACUC subcommittee when visiting their own facilities IACUC Member/PI Conflict of Interest when visiting their own lab (or housing facility) IACUC Member Training – resources available Effective Communication Rounding up the Researchers Which labs to visit (based upon species and type of activity) PI/Laboratory Manager contact for the visit Phone number Meeting time range Maintain the list for easier coordination during USDA inspections & AAALAC International site visits Day(s) before the IACUC site visit: reminders to IACUC members Reminders to PI/researcher contact personnel Have an office member available during the visit to help troubleshoot – if an IACUC member doesn’t show up for the visit, if PI/laboratory contact has questions during the visit (when are they coming to my lab?), etc. Site Visit Team Required: Minimum of 2 voting IACUC members (cannot be a primary member and their alternate) Optional: o PI/Researcher Representative o Facilities/Engineering Representative o Environmental Health & Safety Representative o Building Administrator o Compliance Liaison o Facility Veterinarian o Others? Post-Site Visit Frenzy Managing/tracking deficiencies Minor vs. Significant Communicate deficiencies to whom? Significant deficiencies – AV staff and PI staff immediately? Responsible party for the room, animals, etc. Shared spaces – who is responsible for correcting the deficiency? Documentation – database, emails, etc. Resolution/Corrective Actions Minor vs. Significant Require response by – reasonable time? Methods to confirm resolution/corrective action took place? Trust, but verify Post-Approval Monitoring Review previous deficiencies prior to and send with next SV team for reference Questions for OLAW, USDA, AAALAC 1) …activities, such as routine dosing, weighing, or immunization of animals in laboratories, may be monitored using other methods such as random evaluation… a) Can these visits to be performed by members of the institution, other than an IACUC subcommittee (e.g. compliance liaisons during postapproval monitoring visits), and b) Does this include all non-surgical procedure areas (e.g. CO2 euthanasia stations within PI labs, imaging procedures that may/may not require anesthesia, blood collection)? Questions for OLAW, USDA, AAALAC 2) 3) If the AV and their designee(s) are voting IACUC members, can they serve as either of the 2 IACUC members on the subcommittee performing the visit for areas that are managed by their unit? Similarly, can an IACUC member be either of the 2 subcommittee members performing the visit for: a) their own laboratories? b) animal facilities where their animals are housed? c) laboratories for PIs within their department? d) their department Chair? Questions for OLAW, USDA, AAALAC 4) Repeat Offenders a) When is this considered to be continued non-compliance and potentially reportable…to OLAW (if PHS funded) and AAALAC International (if accredited) and to USDA (if covered species and your institution elects to self-report)? b) 5) When is an item considered to be a program-wide deficiency and potentially reportable…to OLAW (if PHS Assured) and AAALAC International (if accredited) to USDA (if covered species and your institution elects to self-report)? Others?