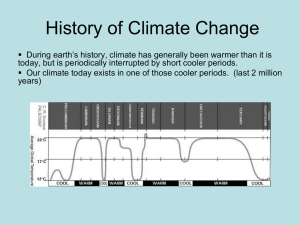
2012 ASCO Highlights: Hematological Malignancies
advertisement

Wei Ai, M.D., Ph.D. June 2012 I. II. III. IV. Lymphoma: bendamustine CLL/SLL: Btk inhibitor Pre-B ALL: Bite biphasic antibody Multiple Myeloma: Carfilzomib National LymphoCare Study 2004 -2007 N = 2728 R + chemotherapy: Regimen Users (%) RCHOP 55.0% RCVP 23.1% R-fludarabine 15.5% Other 6.4% Friedberg, et al., JCO 2009 Newly diagnosed indolent lymphoma or mantle cell lymphoma: - Follicular lymphoma (grade 1-3a), small lymphocytic lymphoma, marginal zone lymphoma, wadenstroms, mantle cell lymphoma (elderly) Stage III and IV Meet criteria for initiating treatment Samantha Mary Jaglowski, et al. The Ohio State University BTK inhibitor, an oral agent, showed promising activity as a single agent in untreated elderly pts (>65 yo) with CLL/SLL (John Byrd 2012 ASCO) - N = 26 - ORR 81%, CR 12% with medium f/u 14 mos Ofatumumab is active in fludarabine- or alemtumumab-refractory CLL/SLL pts: ORR approximately 50% Wierda, et al., JCO 2010 Ibrutinib 420 mg po qd Oftumumab - 300 mg C2, day1 - 2000 mg C2,days 8, 15, 22 - 2000 mg C3, days 1, 8, 15, 22 - 2000 mg C4-8, day 1 only To determine the toxicity of the combination regimen - Tolerability is defined as no more than 1 DLT in the first 6 pts treated for 2 cycles To evaluate ORR at one year: N = 27, including the initial 6 CLL/SLL or Richter’s transformation Two or more prior therapy, including a purine analog- containing regimen More than 10% CD20 expression on CLL cells by flow cytometry Adequate organ functions Ibrutinib combined with ofatumumab is a well tolerated and highly active regimen in patients with relapsed and refractory CLL/SLL Max Topp et al. 80% 15 ug/m2/24 hrs CIV x 4 weeks, 2 weeks off, q 6wk-cycle Topp, et al., JCO 2011 Dose-finding Phase: - Cohorts: 1, 2a, 2b Expansion Phase: Dosing: CIV 4 wks on, 2 wks off, for up to 5 cycles - CR/CRh within the first 2 cycles -> allo Primary Endpoint: CR/CRh within 2 cycles R/R ALL, > 5% blasts in BM Ph+ and relapsed after allo were permitted Selected dose for expansion cohort based on lowest treatment-related AEs (3/5 pts): 5ug/m2/d CIV week 1, then 15ug/m2/d CIV thereafter N = 23 Median Age 31 (21 – 66) Refractory 1 (4%) Relapsed 21 (91%) 1st relapse <= 18 mos 9 (43%) 1st relapse > 18 mos 4 (19%) >= 2nd relapse 8 (38%) Prior SCT 10 (43%) Ph + 2 (9%) T (4,11) 1 (4%) Blasts in BM >60% 12 (52%) 10% - 60% 6 (24%) < 10% 4 (17%) Cytokine release syndrome - Risks: high tumor burden ans without cytoreductive phase - Prevention: cytoreduction 5ug/m2 wk 1 and give Dex for BM>50% CNS Adverse Events: - 3 seizures and 3 encephalopathy: fully reversible - all 6 pts continued at 5 ug/m2 One pt died of fungal infection CR/CRh: 17/23 pts (74%) All but 2 responders achieved molecular remission High remission rate in all pt groups, including Ph+ 13 pts received an allogeneic SCT With a median follow-up of 4.5 months, median duration of response was 8.9 for all cohorts, not yet reached for the expansion cohort Well-tolerated at 5 ug/m2/d followed by 15 ug/m2/d CIV, 4 weeks on, 2 weeks off High hematologic and molecular response rate Median duration of complete hematologic response was 8.9 months Median survival was 9 months Carfilzomib Coming to the Front-line Disease status - High risk, intermediate risk vs low risk - special clinical scenarios: plasma leukemia, renal failure Patient factors - Transplant eligibility - PS and comorbidity Clinical benefit - response and OS - QOL Toxicity and convenience Cost Newly approved second-in-class proteasome inhibitor Well tolerated, no neurotoxicity Overcome bortezomib resistance in vitro Active alone and in combination regimens for relapsed/refractory MM Kuhn et al., Blood 2007, O’Connor Clin Cancer Res 2009, Wang, M et al., JCO 2011 Abstract 8052, Vij, et al., Blood, 2012 CMP carfilzomib, melphalan, prednisone CYCLONE carfilzomib, cyclophosphamide thalidomide dexamethasone CRd carfilzomib, lenalidomide dexamethasone Key Criteria Transplant ineligible >65 yo Transplant eligible Transplant ineligible or eligible Comparative or Parent regimens VMP CyBorD CTD RVD,VTD, VTD Carfilzomib: 20mg/m2 first week, 27mg/m2 thereafter Stringent complete response (sCR) in patients with newly diagnosed multiple myeloma (NDMM) treated with carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (DEX) AJ Jakubowiak,1 K Griffith,2 D Dytfeld,3 DH Vesole,4 S Jagannath,5 T Anderson,2 B Nordgren,2 K Detweiler-Short,2 D Lebovic,2 K Stockerl-Goldstein,6 T Jobkar,2 S Wear, 7 A Al-Zoubi, 2 A Ahmed, 2 M Hussein,8 H Yeganegi,9 R Vij6 1University M of Chicago, Chicago, IL; 2University of Michigan Comprehensive Cancer Center, Ann Arbor, MI; 3Poznan University of edical Sciences, Poznan, Poland; 4John Theurer Cancer Center, Hackensack, NJ; 5Mount Sinai Medical Center, New York, NY; 6Washington University School of Medicine, St. Louis, MO; 7Multiple Myeloma Research Consortium, Norwalk, CT; 8 Celgene, Inc, Summit, NJ; 9 Onyx Pharmaceuticals, South San Francisco, C Objectives Primary • Phase 1: MTD of CRd • Phase 1/2: rate of ≥nCR Secondary • Overall response rate (≥PR) • TTP, DOR, PFS, and OS • Tolerability and toxicity • For transplant candidates, evaluate the impact of CRd on stem cell mobilization • Evaluate prognostic factors and markers of response 4 Eligibility Key inclusion criteria • Newly-diagnosed MM requiring first-line therapy1 - Transplant-eligible and -ineligible • Measurable disease per IMWG Criteria1 • ECOG performance status 0-2 Key exclusion criteria • Grade 3/4 peripheral neuropathy • ANC <1.0x109/L, Hgb <8.0 g/dL, platelets <75,000/µL • Creatinine clearance <50 mL/min or serum creatinine ≥2 g/dL • Serious co-morbidities 1. Durie BGM, et al. Leukemia. 2006;20:1467-1473. 5 Treatment Schema CRd Induction Transplanteligible and -ineligible CRd Cycles 1-4 patients CRd Maintenance CRd Cycles 5-8 Transplant-eligible ≥PR ASCT CRd Cycles 9-24 Lenalidomide (off protocol) LEN Cycles 25+ Until disease progression or unacceptable toxicity Stem cell collection • Assessments on D1 and 15 of C1 and D1 thereafter using modified IMWG Criteria with nCR Cycles 1-8 • CFZ 20-27-36 mg/m2 Days 1-2, 8-9, 15-16 1 • LEN 25 mg Days 1-21 • DEX 40 mg weekly Cycles 14, 20 mg weekly Cycles 5-8 Cycles 9-24 • CFZ on Days 1-2 and 1516 only • CFZ, LEN, DEX at last best tolerated doses 1. Jakubowiak AJ, et al. Blood. 2011;118: abstract 631. Cycles 25+ • LEN at last best tolerated dose 6 Best Response Median 12 cycles (range 1-25) ≥PR ≥VGPR ≥nCR sCR 100 98 80 60 40 81 62 42 20 0 All patients N=53 There was no difference by disease stage and cytogenetics 9 Progression-free Survival Median follow-up of 13 months (range 4-25) 2 patients progressed All patients with sCR have maintained response for median 9 months (range 1-20) 12 Updated Toxicity of CRd Induction Thrombocytopenia Anemia Neutropenia Grade 3/4 Any grade Hyperglycemia Edema Hypophosphatemia Fatigue Muscle cramping LFTs Rash Diarrhea Infection Phlebitis Peripheral neuropathy Dyspnea DVT/PE Renal Constipation Mood alterations 0 20 40 Patients (%) 60 80 100 • Toxicity for cycles 1-8 is similar to previously reported1 • Limited dose modifications 1. Jakubowiak AJ, et al. Blood. 2011;118: abstract 631. 13 CMP carfilzomib, melphalan, prednisone CYCLONE carfilzomib, cyclophosphamide thalidomide dexamethasone N = 24 CRd carfilzomib, lenalidomide dexamethasone Transplant ineligible >65 yo Transplant eligible Transplant ineligible or eligible VMP CyBorD CTD RVD,VTD, VTD 31(89%) 1 (3%) 23 (96%) 7 (29%) 14 (40%) 16 (46%) 11 (46%) 5 (21%) 52 (98%) 33 (62%) 22 (42%) 11 (20%) 10 (19%) 9 (17%) N = 35 Comparative or Parent regimens ORR - CR - sCR - nCR - VGPR - PR N = 53 Highly active as a first-line treatment for MM The quality of response seems improved in some studies Tolerability seems improved with minimum peripheral neuropathy, although comparison with SQ bortezomib remain to be investigated Lymphoma and CLL/SLL The Stil trial established R-Benda as the preferred first-line treatment for FL and MCL Ofatumumab + ibrutinib (Btk inhibitor) is highly active in relapsed/refractory CLL/SLL Acute Leukemia Blinatumumab (Bite biphasic antibody) is highly active in relapsed/refractory ALL Multiple Myeloma Carfilzomib is moving to the front line CHOP-R N =253 BR N = 261 Neutropenia 69% 29% lymphopenia 43% 74% Grade 3/4 Topp, et al., JCO 2011