IRB & Regulatory Agencies
advertisement

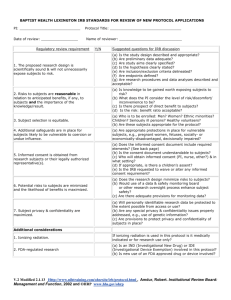
Institutional Review Board for Human Research (IRB) Human Subject Protections Susan Sonne, PharmD, BCPP Chair, MUSC IRB II Nuremberg Code first major control on research in any nation prescribed 1948 as part of trial of a Nazi Physician willfully harmful research on unwilling human subjects Jewish Chronic Disease Hospital Study • July 1963 • Injection of live cancer cells into 22 patients • No written consent • Some verbally informed – “involved in experiment” Never told being given live cancer cells • Guilty of fraud, deceit, unprofessional conduct The Declaration of Helsinki Produced 1961, adapted 1964 Informed consent = ethical research Basis for FDA policy Willowbrook State School Study began 1956 Institution for “mentally defective” children Study designed to contribute to understanding etiology of infectious hepatitis and test effects of gamma globulin in preventing disease. First subjects fed extracts of infected stool Public attention 1971 National Research Act of 1974 Created National commission for the protection of Human Subjects of Biomedical and Behavioral research. Charge was to: Identify basic ethical principles that should underlie conduct of biomedical and behavioral research involving human subjects More specifically, consider Boundaries between research and practice Role of assessment of risk-benefit Selection of subjects Nature and definition of informed consent Result was 1976 Belmont report Good Clinical Practice Why have this training? The Tuskegee Syphilis Study 1932 to 1972 399 African American Males were denied treatment for syphilis Study conducted by U.S. Public Health Service From President Clinton "Although these regulations [Protection of Human Subjects, Code of Federal Regulations, Title 45, Part 46} provide the framework for protecting human subjects in research, we must exercise constant care and ensure that these regulations are strictly enforced by departments and agencies. Therefore, I direct each department and agency of Government to review present practices to assure compliance with the Federal Policy for the Protection of Human Subjects and to cease immediately sponsoring or conducting any experiments involving humans that do not fully comply with the Federal Policy." -Bill Clinton, 1994 “The people who ran the study at Tuskegee diminished the stature of man by abandoning the most basic ethical precepts. They forgot their pledge to heal and repair. They had the power to heal the survivors and all the others and they did not. Today, all we can do is apologize….” – Bill Clinton, 1997 OHRP Suspensions During the last 6-7 years, OHRP has suspended all human research at the following institutions: – University of Illinois, Chicago Campus – University of Colorado – Duke University – Johns Hopkins – University of Pennsylvania – And others General Rule for Human Subject Protection Any element of research? Yes Undergo review IRB Purpose To protect the rights and welfare of human research subjects Authority to approve, require modification and disapprove any research involving human subjects Institutional Review Board • responsible to verify 1. Safety 2. Integrity 3. Human rights 4. Public reassurance 5. Scientific content The Belmont Report 3 Basic Principles for Protection of Human Subjects: – Respect voluntary informed consent privacy protections for vulnerable populations – Beneficence – Justice Beneficence Persons treated in an ethical manner Protecting them from harm Helping to secure their well being Justice • Who ought to receive the benefits of research and bear the burden of research? • Equals ought to be treated equally • Fairness in distribution • What is deserved Criteria for Approval Risks are minimized Reasonable risk:benefit ratio Equitable selection of subjects Informed consent obtained appropriately Informed consent adequately documented Monitor data to insure safety Protect confidentiality No coercion Informed Consent • Essential to ethical conduct of clinical investigation • Potential subject chooses whether or not they will participate • Obtained after full information is given and understood • Explanation of study objective, potential benefits, risks, inconveniences, subject’s rights and responsibilities Informed Consent –Introduction –Purpose of the Study –Procedures –Potential Risks and Discomforts –Anticipated Benefits to Subjects –Anticipated Benefits to Society –Alternative to Participation –Emergency Care and Compensation for Injury –Payment for Participation –Financial Obligation –Privacy and Confidentiality –Participation and Withdrawal –Rights of Research Subjects –Signatures The Mountain of Responsibility •Protecting Participants •Implementing Study •Reporting Accurately Investigator Obligations • Conducts a clinical investigation • Responsible for All procedures conducted All data collected • May delegate work in conducting study but they retain responsibility Investigators – and by delegation all study staff responsibilities • To protect the rights of participants • To obtain informed consent from each participant • To keep specific records and issue specific orders • To assure that the IRB is provided information for initial and continuing review of the study Useful Regulatory/Policy Material MUSC IRB http://research.musc.edu/ori/irb/home Office for Human Research Protections http://www.hhs.gov/ohrp/ Food & Drug Administration http://www.fda.gov/oc/ohrt/irbs/default.htm