Good Regulatory Practice-June2012-Draft
advertisement

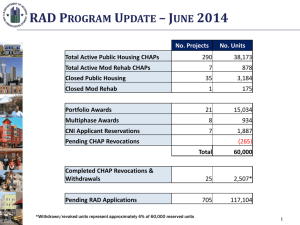
MEDICINE REGULATION REGULATORY DEPARTMENTS GOOD REGULATORY PRACTICE SAAPRA 1 June 2012 OVERVIEW Development of Legislation Medicines Regulation and Regulators Regulatory Affairs Department (RAD) Good Regulatory Practice (GRP) Development of Medicines Legislation Typically medicine regulations have been developed ‘after the fact’ Triggered by unwanted and sometimes disastrous events Information and knowledge on the use of medicines increases exponentially Result - Medicines legislation proliferates Development of Legislation USA 1846 – 1848 Mexican-American War American troops supplied with substandard medicines 1848 – Import Drug Act passed 1901 – concern over unsanitary condition in meat packing industry and also quality of medicines Medicines legislation outgrowth of food legislation Development of Legislation 1902 – 12 children died from contaminated diphtheria toxin in St Louis Result - Biologics Act of 1902 Demanded licensing of biological products and facilities 1902 – 1907 Study on safety of food additives with human volunteers Led to - 1906 Pure Food and Drugs Act First federal drugs law Development of Legislation Prohibited mislabelling and adulteration of medicinal products and Introduced USP and NF as official standards Legal system still allowed for unacceptable bizarre practices false therapeutic claims 1912 - US vs Johnson case – promoters of “Dr. Johnson’s Mild Combination Treatment for Cancer” challenged 1912 Act amended –prohibited labelling with false therapeutic claims Development of Legislation 1937 – Massengill Company place throat lozenge on market Sulfanilamide dissolved in diethylene glycol (common car antifreeze agent) 107 people died (mostly children) Legislators acted rigorously to prevent reoccurrence 1938 – Food, Drug and Cosmetic Act passed Medicine could only be marketed after 60 days if no FDA objections Development of Legislation Required proof of safety and efficacy 1951 – another amendment to Act Divided products into OTC and prescription requiring professional supervision 1962 amendments made proof of efficacy mandatory Introduced GMP Development of Legislation Further amendments over 20 years such as 1983 Orphan Drugs Act – for marketing and commercialisation of medicines to treat rare diseases 1990 Nutrition and Labelling Act 1984 Drug Price Competition and Patent Term Restoration Act 1996 Generic Drug Enforcement Act Development of Legislation 1988 Prescription Drug Marketing Act To ensure that medicines purchased by consumers are safe and effective, and To avoid the unacceptable risk to American consumers from counterfeit, adulterated, misbranded, subpotent, or expired drugs. Additionally Guidance documents to be taken into account by industry Other reform bills constantly under consideration Development of Legislation EUROPEAN UNION (EU) Germany and other countries originally focussed on pharmacies only No Marketing Authorisation required for industrially produced medicines 1961 registration introduced to determine what was on market – notification process only In Germany 55 000 medicinal products on market at time Development of Legislation Beginning 1960s sleeping pill Contergan (thalidomide) caused birth defects Taken by mothers in early stages of pregnancy Children born without arms hands starting at the shoulders Called ‘flipper babies’ Development of Legislation An estimated 10 000 children were affected in Europe 1968 appetite suppressant Menocil (aminorex) caused many deaths – withdrawn from market Led to implementation of medicines legislation in UK and other EU member states Today legislation in EU member states mirrors EU Directive Development of Legislation SOUTH AFRICA 1965 Medicines and Related Substances Control Act published and first medicines called up in 1968 2002 Medicines and Related Substances Act amended making provision for inter alia licensing of manufacturers, wholesalers, distributors, etc. 2008 Amendment Act 72 introduced SAHPRA Latest draft amendment makes provision for the structures required for the new juristic body International Conference on Harmonisation(ICH) ICH began to take place in 1989 Project of both Regulatory Bodies and pharmaceutical industry from EU, Japan and United States Harmonisation the major factor Goal - to expedite development and approval processes for medicinal products Does not compromise safeguards on Q/S/E World Health Organisation (WHO) An intergovernmental organisation 166 member states within the Charter of the United Nations Activities include support for ministries of health concerning development of methods for assessing quality, effectiveness and efficiency Publications cover: essential drugs drug policies quality control ethical guidelines safety assessment, drug research and development and laboratories Medicines Regulation and Regulators Medicine legislation makes provision for Regulatory Authorities or Bodies Have developed and continue to develop legislation This had and continues to have a significant effect on pharmaceutical industry Medicines Regulation and Regulators Common misunderstanding – that Regulators register our medicines They keep medicines off the market unless the applicant can prove quality, safety and efficacy Ensure compliance with legislation Control the use and minimise the abuse of medicines Re-evaluate the medicines on the market Regulatory Affairs Departments (RAD) Why have companies established Regulatory Affairs Departments ? Simple answer – because there are regulations and regulators To understand and fulfil regulators’ needs as environment has become more and more complex Regulatory Affairs Departments (RAD) Mistakes cost a lot of money Worst mistake – calling in RA after plans have been finalised Pitfalls of product development: Not involving RA in plans Suppressing critics in the company – they are valuable in identifying problems Hiding critical issues chances good reviewer will spot them Telling all you know – leads to confusion Regulatory Affairs Departments (RAD) - Trying to make it perfect – a lot of time will be lost Doing all the studies as early as possible e.g. marketing studies Using in-house methods to structure the documentation – adapt to Regulator’s structure Regulatory Affairs (RA) RA is usually recognised as having three basic functions: The outlet of the company to Regulatory Bodies The interpreter of regulations to companies The influencer of new regulations Makes RA the interface between companies and Regulatory Bodies and Key player in the product development and maintenance process Activities of the Regulatory Affairs Department (RAD) Activities depend on each company’s structure and organisation Start at the initial development of medicinal products Continue through until the launch of the product Steer and maintain an application through the legislative framework - allows a Regulatory Authority to reach a scientific decision Once launched, the RAD fully involved in Marketing Authorisation (MA) maintenance and post-marketing activities Activities of the RAD Product Development RAD should be involved in the creation and evaluation of all aspects of research and development plans Advise departmental heads and project managers on the requirements and any upcoming legal changes with potential impact for registration RAD should give advice for optimising development plans By an accurate interpretation of the requirements of the existing guidelines Ideally should be done within the scope of a Regulatory Strategy Document Activities of the RAD Product Development Ideally RAD should be able to liaise with Regulatory Authorities on any scientific aspect of: – future Marketing Authorisation (MA) – dossier and co-ordinating with the other departments – the briefing document including the questions and company’s positions On our wish list! Activities of the RAD New Registrations RAD responsiblities: proposing the best registration strategy taking into account the possible registration procedures the impact of intellectual property rights the peculiarities of the product the scientific content of the different part of the dossier RAD preparation of Regulatory Strategy Document (RSD) containing all the essential global regulatory aspects of product development including scientific advice meeting with the Regulatory Authority Activities of the RAD New Registrations RAD – ensure all activities related to obtaining registration comply with existing legislation i.e. – laws, regulations, directives and guidelines – need to be proactive RAD - should establish ethical, practical, technical and regulatory standards: laid down in policies and SOPs specify the responsibility of each staff member involved describe the process ensure content of dossiers in compliance with existing legislation and standard needed to obtain registration of the product Activities of the RAD New Registrations RAD responsiblities: – writing, co-writing, editing and/or authorising all documentsfor use in registration dossiers or communication with the Authority e.g. manuscripts, expert reports, method of use, standard advice for patients and labels – should strive for world-wide implementation of harmonised prescribing information – should be responsible for the accurate planning and co-ordination of compiling the dossiers – Take into account the possibilities for electronic submission of the dossier or parts of it especially in terms of submission of eCTDs Activities of the RAD New Registrations – – – Ensuring administrative validation Chasing up the dossier throughout the assessment and Anticipating the possible questions from the Regulatory Authority in order to optimise the timing, quality of the answer and Marketing Authorisation (MA) approval and package insert (PI) wording Queries from the RA should be answered in a consistent manner and within the time limits set by the agency when indicated, according to internal guidelines Status reports should be issued regularly in order to provide information on the world-wide registration situation Activities of the RAD Registrations – – – – – – submission strategies (timing, responsibilities, free sale certificates and answers to the Authority, questions, intellectual property dossier updates (variations and safety) dossier renewals Periodic Safety Update Reports (PSURs) planning and a contingency plan must address the proposed labelling with Marketing and Sales the impact on discussion with pricing and reimbursement authorities Activities of the RAD Maintenance of Existing Registrations All existing registrations should be carefully maintained and regularly updated to reflect the current standards and knowledge Variations and change control: – Management of change control forms are an important element of GRP - Such changes include: o Product data or specifications o Manufacturing of analytic methods o Facilities or suppliers as well as line extensions o Additional indications, etc. All such changes have to be communicated to the RA in accordance with the respective legal requirements affected Activities of the RAD Maintenance of Existing Registrations Queries raised by the RA to ensure regulatory compliance RAD and quality assurance department should liaise closely on all aspects affecting variations and change control Responsibility of RAD, Responsible Pharmacist and QA (Quality Assurance) to ensure manufacturing and QC (Quality Control) comply at all times with the registration dossier RAD should assure regulatory compliance by the manufacturer, packager and marketing department Activities of the RAD Maintenance of Existing Registrations Changes to PI and/or PIL Changes might be initiated by marketing or medical department Can also be requested for new safety data RAD and medical / pharmacovigilance c liaise closely for the preparation and timely implementation once the revised wording is approved RAD in close collaboration with the Drug Safety department and a Qualified Person for Pharmacovigilance Do PSURs planning on a yearly basis for each product Collect all the information needed for their submission, taking into consideration post-approval commitment or follow-up measures Activities of the RAD Maintenance of Existing Registrations It is the duty of RAD to ensure activities related to post marketing studies are carried out in compliance with and reported according to, existing legislation. to inform the Regulatory Authorities of any pharmacovigilance issue and to implement and file the necessary data into the official documents this will be done in close co-operation with the medical department and Qualified Person for Pharmacovigilance Activities of the RAD Regulatory intelligence – Objective 1 RAD should – have a system in place to ensure the tracking of all the versions, either approved or a draft for comment, of regulations guidelines and concepts papers on an on-going basis review relevant world-wide legislation, guidelines, discussion papers, codes of conduct, etc. interpret the scope and possible consequences arising from such legislation and codes and inform the company accordingly(may affect the activities of the company) ensure and co-ordinate the necessary activities arising from changes to ensure that compliance with regulations will be met. Activities of the RAD REGULATORY INTELLIGENCE - Objective 2 RAD should – comment on new draft guidelines / draft legislation and take an active role in participating in the outcome of final regulatory comments. for this purpose preferably join pharmaceutical associations to represent the needs of pharmaceutical industry on a higher level. Good Regulatory Practice (GRP) Problem today: Insufficient quality submissions to the Regulatory Authority Industry and Regulator perceive each other as opponents rather than partners We are like opposite sides of the same coin -neither side detracts from the value of the coin but rather together give it its character and identity. Good Regulatory Practice (GRP) Present system based on mutual mistrust Involves high cost i.t.o. time, manpower, rejects on both sides Quality cannot be added by regulations, guidelines, etc. Without any real dossier quality improvement timelines cannot improve Good Regulatory Practice (GRP) Could GRP be the solution to the problem? Good Regulatory Practice (GRP) What is GRP? Establishment of a quality system Involves both industry and Regulator About trying to achieve quality by less rather than more control About producing quality in the first place Based on sound science, coupled with organisational ability Good Regulatory Practice (GRP) What are the goals of GRP? Efficiency: quick and qualified decisions on Q/S/E of products Effectiveness/productivity: effective use of resources, costeffectiveness Results: achieve and preserve an image of high standing Good Regulatory Practice What are the advantages of GRP? Quality medicines available to patients in timely fashion Because global picture is seen and Entire process is designed to produce quality Good Regulatory Practice How? Decide on goals What must be done by whom and how Write it down Adhere to it Watch the results and if necessary modify the system Good Regulatory Practice(GRP) A pre-requisite to GRP - the communication of all relevant information, including its evaluation and relevance for the existing product, to ALL interested parties within the organisation Pivotal to GRP is the need to keep abreast of world-wide legislation including potential changes and The interpretation of possible consequences in the event of failure to meet requirements Good Regulatory Practice(GRP) • • • The involvement of GRP in the concept of Total Quality Management (TQM) is essential from initial development phase of the product and continues for its entire life Efficient RAD organisation and working methods are mandatory RAD skills for communication and communicating information to other departments - a key parameter to ensure compliance with regulatory requirements Good Regulatory Practice (GRP) – – – Implementation of GRP essentially contributes towards continuous Total Quality Assessment of all aspects of regulatory affairs and is The essential link between each discipline in Total Quality Management Increasing complexity of regulatory requirements especially as RADs operate numerous interfaces within a pharmaceutical company Good Regulatory Practice (GRP) GRP Guidelines define the role and position of the RAD within the organisation Appropriate and effective management of the regulatory process is mandatory for: – Bringing a medicinal product to the market and keeping it there – In compliance with legal, scientific, ethical and administrative requirements – To ensure the compliance of the relevant company’s activities to the local and global regulations in terms of official and company regulations PERSONNEL There should be sufficient personnel at all levels within the organisation with the: Ability Education Training Experience and Appropriate professional skills to perform the tasks assigned to them All personnel should be trained regularly in their skills to ensure they possess sufficient skill and knowledge of the procedures and policies of the organisation Ideally, all graduate personnel should have a multidisciplinary background PERSONNEL Ideally, all graduate personnel should have: – multidisciplinary background – scientific expertise – communication and negotiation skills – regulatory knowledge – planning capacity – be able to work in teams – to organize multidimensional projects – to work in a multidimensional manner Standard operating procedures should be designed to deal with communication and flow of information PERSONNEL The responsibilities of the Responsible Pharmacist (RP) and the Qualified Person for Pharmacovigilance (QPPV) in relation to the RAD should be clearly defined These responsibilities should be explained and written into the job description for each person together with any training and education required on product registration Key personnel in responsible positions should be accountable for: – authorizing procedures and tasks and – having adequate supporting staff – persons should be designated to deputise for them in their absence PREMISES Premises should be designed and maintained in good order To provide sufficient space to suit the activities being carried out Should allow efficient work flow Should permit effective communication and supervision Physical and non-physical working conditions: – Ergonomics – Environmental factors – Stress All necessary equipment for the activities to be performed efficiently should be provided – personnel should be instructed in the proper use of equipment: – Computer hardware and software – Printers and copiers – Archives and means of communication QUALITY ASSURANCE (QA) A good quality management system (QMS) should be set-up by RAD for all the activities under its responsibility QUALITY ASSURANCE Procedures Procedures should be laid down in writing in standard operating procedures Authorised by appropriate staff and communicated to the relevant personnel This should be readily available and be checked and updated regularly SOPs should be adapted / renewed in the case of new or amended standards SOPs should be established on how to implement relevant legislation and codes and the consequences for the organisation’s policy RAD should develop policies for situations in which changing legislation and codes make it necessary to adapt to it QUALITY ASSURANCE Procedures Procedures should cover the different areas in RA to: – Specify responsibilities and organisation o regulatory development plan o development of PI and PIL o preparation of CTD o compilation of dossier o change control o preparation of response documents o maintenance of registration o preparation of renewals, etc. QUALITY ASSURANCE Self-Inspection Regular self-inspections should be performed by RAD in cooperation with QA to – check and ensure compliance with the relevant regulations by staff at all levels check the compliance with the procedures and adapt the procedure according to current practices QUALITY ASSURANCE Training An initial training program should be in place for new employees to ensure a good understanding and therefore compliance All RAD staff should be regularly trained in procedures to ensure good understanding and therefore compliance Each new procedure or update of existing procedure should also lead to a specific training on the changes implemented Understanding of all these aspect of the procedure should preferably be assessed by knowledge control A tracking system of the annual training should be organized DOCUMENTATION Good documentation is essential for the whole organisation and especially the RAD All documentation should be prepared with great care and clearly written to prevent errors that can arise from oral communication DOCUMENTATION Documents should contain all the information necessary for proper use: – Title, type and objectives should be unambiguous and clearly stated in SOPs – All documentation should be reviewed regularly and kept up to date – Amendments should be dated, authorized and signed by the appropriate personnel DOCUMENTATION An appropriate system should be in place to: o ensure traceability of documents and their different versions o answers to the Regulatory Authorities o changes in PI o variations etc. All documentation should be securely stored but readily accessible to RA personnel When prepared or stored electronically validation processing programmes should be used DOCUMENTATION Data should be protected against loss or damage e.g. – Use of backup procedures – Only authorised personnel should be allowed to enter or change data ARCHIVING A good archiving system is mandatory: – It should be described in a procedure – Describe, both the documentation and electronic documents, – The archiving plan, – The management of the different versions, – The answer to questions from Regulatory Authorities, – The traceability and the measures taken to ensure regular backup. COMMUNICATION Due to the multidisciplinary activities under the responsibility of RAD close relationship with almost all the departments is needed: – Pre-clinical – Medical – Pharmacovigilance – Production – Quality Control – Quality Assurance – Marketing and sales etc COMMUNICATION RAD skills for communication and communicating information to other departments, Regulatory Authorities, Professional Associations Communication - Key parameter to GRP in terms of compliance with regulatory requirements, lobbying, negotiation, effective relationship with external bodies Checklist for Performance of RADs Perceives disciplines and Regulators as partners, not enemies Establishes/maintains efficient contact with Regulator Works proactively Proactive in product development/maintenance teams Market orientated and customer focussed Submits dossiers of sufficiently high quality in a timely fashion to obtain and maintain registrations Maintains a quality system Advice Make your mission in the organisation clear. Make your voice heard. You are one of the most valuable team members if allowed to live up to full capacity. Suggested Reading Good Drug Regulatory Practices A Regulatory Affairs Quality Manual Helene I. Dumitri Over to you! MRA Regulatory Consultants 381 Rossouw Street Murrayfield Pretoria Tel: +27 (0)12 803-6223 allison@mra-regulatory.com henriette@mra-regulatory.com robyn@mra-regulatory.com