U.S. Trade Policy and Nanotechnology
advertisement

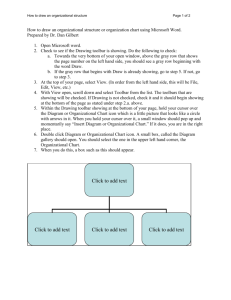
U.S. Trade Policy and Nanotechnology Jeffrey Weiss Senior Director, Technical Barriers to Trade Office of the United States Trade Representative Washington, DC September 22, 2011 Overview Primer on Key International Trade Rules and the Role of the USTR USG Nano Principles Current/Future Issues in Trade & Nanotechnology 2 Overview of Key Trade Rules Relevant to Nanotechnology The WTO Agreement on Technical Barriers to Trade (TBT Agreement) provides disciplines on product standards, including labeling and packaging, and related conformity assessment procedures (e.g., testing, inspection, certification, registration) to ensure they don’t create “unnecessary” TBTs Other relevant agreements include the GATT (e.g., prohibition on import/export restrictions, nondiscrimination) and the SPS Agreement (e.g., food safety, animal health, pest control) 3 What products/issues are potentially covered by TBT? Lawnmowers Chemicals/hazardous substances Auto safety/emissions Mobile phone batteries Medical devices Clothing, footwear Baby food containers Snack foods Agricultural product standards IT products Packaging rules Halal/organic standards Cosmetics Wine/distilled spirits Pharmaceuticals Cigarette lighters Toys/product safety Energy drinks Nutritional supplements Infant formula Encryption Plugs and sockets Labeling (e.g., GE) Solar panels I.e., all industrial and agricultural products 4 Key TBT Agreement Questions Questions we ask when determining whether a foreign measure may raise trade concerns: Does the measure treat an imported product less favorably than a like domestic product or a like imported product from another country? Is the measure “more trade restrictive than necessary to fulfill a legitimate objective” (e.g., health, safety, environment, consumer protection)? Relevant factors to consider include available scientific and technical information and the intended end uses of a product Does the measure use a relevant international standard as a basis, unless such standard is ineffective/inappropriate to fulfill the objective? Was the measure developed in a transparent manner (i.e., notified to the WTO, with a meaningful opportunity for comment provided and comments taken into account)? Was a reasonable period of time for implementation provided so that suppliers have sufficient time to comply before entry into force? 5 USG Organizational Structure USTR has statutory lead on development, implementation, and coordination of U.S. trade policy, including the negotiation and enforcement of trade agreements (e.g., WTO, NAFTA, TPP, mutual recognition agreements) Trade Policy Staff Committee (TPSC) 6 Trade Policy Staff Committee (TPSC) Subcommittee on TBT Interagency mechanism for developing, coordinating, and implementing U.S. trade policy Determines whether to raise trade concerns about particular foreign measures and what arguments to make (as well as how to respond to foreign concerns about U.S. measures) USTR chairs the Subcommittee and coordinates the policy development process Regulators participate in the TPSC, helping to ensure that no arguments are made against foreign measures that could be used to undermine U.S. health, safety, environmental, etc. requirements, and the ability of U.S. (Federal and State) regulators to protect U.S. citizens, patients, and consumers Decisions are taken by consensus 7 USG Organizational Structure (continued) Relationship with Congress Official USG Advisory Committees and other stakeholders States 8 USTR’s Role in Implementing the TBT Agreement in the United States Participation in OMB/OIRA process for significant rulemakings Statement of Administration Policy (SAP)/letters Informal means (e.g., direct contact with regulators, legislators, stakeholders) 9 USG Principles for Regulation of Nanotech and Nanomaterials Origins U.S.-EU High Level Regulatory Cooperation Forum Past experience with biotechnology Need to engage internationally Emerging Technologies IPC Co-chaired by OMB/OIRA, OSTP, USTR Participation of key regulators Developing a coherent approach 10 USG Principles for Regulation of Nanotech and Nanomaterials Key Conclusions in Principles Document Objectives: Need to ensure we can regulate effectively for health, safety, environment while also promoting job creation, competitiveness, exports, and economic growth and not stifling innovation Existing statutes are sufficient Single definition not necessarily useful Focus not just on size, but on novel properties 11 USG Principles for Regulation of Nanotech and Nanomaterials Importance of transparency, public participation in rulemaking Communication with the public is key Builds trust/confidence Must be clear, accurate, realistic, informative Convey benefits and risks without implying that nano is intrinsically benign or hazardous 12 USG Principles for Regulation of Nanotech and Nanomaterials Regulation should be science/risk-based (importance of scientific integrity) Base decisions on potential benefits and costs Seek coordinated/consistent approach across the USG Coordination with States, stakeholders, international community (e.g., research, policy) Seek and develop adequate information Approach to evolve over time as we learn more, need to maintain flexibility to adapt 13 Current/Potential Trade Issues Involving Nanotechnology Labeling developments Actual measures (EU, Korea) ISO/CEN work on nanolabeling Issues involving collection/sharing of data Standardization issues Work in progress in many fora Risk of unnecessary divergence (e.g., definitions)? Are these standards being used? Export Promotion 14 THANK YOU FOR YOUR ATTENTION! Jeff Weiss Senior Director, Technical Barriers to Trade Office of the United States Trade Representative 1-202-395-4498 Jeff_Weiss@ustr.eop.gov 15