SAP-MOP Geneva 2012_
advertisement

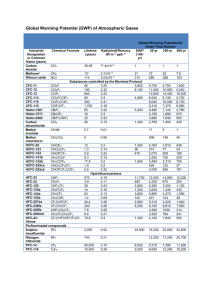
110 Chapter 1, Figure 1-1, 2010 SAP Report NOAA 100 AGAGE 90 1990 1995 2000 2005 Emission or Production (Gg/yr) Global Surface Mixing Ratio (ppt) WMO/UNEP (2011) Carbon Tetrachloride (CCl4) 300 Chapter 1, Figure 1-5, 2010 SAP Report Rate of change E 200 100 2010 0 1985 1990 1995 2000 2005 2010 Carbon tetrachloride (CCl4) continues to decrease in the atmosphere … but its abundance is not consistent with reported emissions and known lifetimes. “Bottom-up” emissions derived from data reported to UNEP are highly variable and on average appear smaller than ”Top-down” inferred from observed trends. Discrepancy (~ 40 Gg per year): Cannot be explained by the lifetime. CCl4 lifetime, = 28±5 years. Errors in reporting, or errors in analysis of reported data, possible illegal prod. Unknown sources or poorly estimated sinks Emission or Production (Gg/yr) New information 300 Chapter 1, Figure 1-5, 2010 SAP Report 200 Rate of change E 100 0 1985 1990 1995 2000 2005 2010 Atmospheric lifetime will increase from 35 years (WMO, 2011) to approximately 50 years. ocean= 94 years, soil=∞ 1 1 atm 1 ocean 1 soil Total lifetime increases from 26 years to about 33 years - ~ the lower bound in WMO (2011) Fraser et al. (2013) estimate that global CCl4 emissions from landfills could be 8-12 Gg/yr. Fraser et al. also suggests there may be some small emission from H2O chlorination Any industrial procedure that uses chlorine in association with organics is likely to produce at least some CTC. An example is the chlorination of carbon monoxide to produce phosgene (COCl2), which is used on a large scale in production of isocyanates, the precursors of polyurethanes. CCl4 summary • A revision of the lifetime will reduce the “topdown” emission estimate by approximately 10-20 Gg/yr • Estimates of global legacy emissions are approximately 8-12 Gg/yr, revising upward the “bottom-up” emission estimate • The 40 Gg/yr emission budget gap between the “top-down” and “bottom-up” estimates has been narrowed, but not quite closed. ODP and GWP of proposed CFC: R-316c • Two isomers • Not clear if the use is for only onecould be a mixture • Atmospheric lifetime and properties are not very different for the two isomers Based on work done at NOAA Boulder: J. B. Burkholder, V. Papadimitriou, M. McGillen, A. Jubb, S. Smith, B. Hall, R. Portmann Work not yet-peer reviewed. To be published. The photolytic loss of RC-316c has been evaluated by laboratory studies • Gas phase reactions in the troposhere too slow to contribute o Mainly lost in the stratosphere: UV photolysis in the stratosphere is the major loss process o O(1D) reactions contribute in the stratosphere • Similar to CFC-12 and 113 • Slightly higher cross section in the key “window” region: 190210 nm • Other tropospheric loss processes may contribute a little Lifetimes and ODP 2D model calculations using laboratory data Molecule Lifetime, yrs CFC-11 58 CFC-12 102 N2O 122 R-316c 81 Consistent with simple scaling: 0.54 rel to CFC-11 0.41 rel to CFC-12 2nd model estimated 0.5 for an ODP ODP 1 0.97 0.46 R-316c is a potent ODS with an ODP of approximately 0.5 IR Cross sections and GWP Based on laboratory data and calculated atmospheric lifetime, the GWP has been calculated. Molecule 20-yr GWP 100-y GWP 500-y GWP CFC-11 6730 4750 1620 CFC-12 11000 10900 5200 289 298 153 4340 4300 2050 N2O R-316c R-316C is a potent greenhouse gas, roughly half as much as CFC-12 and comparable to CFC-11