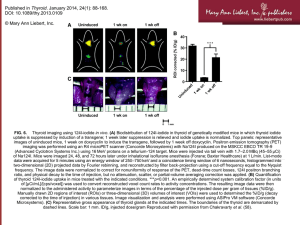
iodine 131/125 - California State University, Long Beach
advertisement

By Pablo Barillas May 3rd 2011 Dr. Mason Both I-131 and I-125 are radioactive isotopes of naturally occurring I-127. I-131 is highly radioactive and gives of energy in both beta and gamma radiation. I-131 has a half-life of 8 days and decays into stable Xenon 131. Created by nuclear fission of either uranium or plutonium. I-125 has electron capture radiation decay where a proton will capture an electron and give off a neutrino. I-125 has a half-life of 59 days will decay into Tellurium 125. I-125 is created by the electron capture decay of Xenon 125. Iodine as a molecule is nonpolar and is highly soluble in non polar organic solvents. Due to its nonpolar nature, it does not dissolve well in water. Solubility is aided by iodide ions already in the water, such as from HI, KI etc and will form the triiodide ion, I3− Iodine is easily oxidized and easily reduced as well. I-131 was discovered by Glenn T. Seaborg and John Livingood at the University of California Berkeley in the late 1930's. I-131 was a huge issue postWWII both in Japan and nuclear test areas in the USA. Currently I-131 is a source of major concern at the Fukishima nuclear plant. I-131, even though harmful in large exposures, has some medicinal uses. I-131 is currently used as treatment for hyperthyroidism by destroying tissue with radiation. Sometimes also used as a radioactive label, but not often since it will destroy tissue in the process. I-125 which is less harmful has more medicinal uses than I-131. I-125 is used in brachytherapy which aids in the destruction of cancerous tissues. I-125 is also used in radiology by tagging antibodies in a radioimmunoassay. I-131 is a nuclear fission product so anywhere fission takes place, I-131 can be found. Fuel rods in nuclear reactors can crack and release I-131 into the surrounding cooling water, which can be released into the environment. Another major source of I-131 is nuclear waste, if not correctly sealed will leak into the environment. A stated before, atomic bomb testing released a lot of I-131 into the atmosphere. Industrial, medical and university waste can also be sources of I-131. I-131 is easily assimilated into the food web by uptake in primary producers such as seaweed. Shellfish can then bioaccumilate I-131 by filter feeding. Vertebrates can absorb I-131 readily by the thyroid gland. Due to radiation, I-131 will lead to tissue destruction and cancer. As stated before, ingestion is the main mode of entry for most organisms. If there is a recent explosition or emission of radioactive products, I-131 maybe inhaled as well. Once in the body, the sodium-iodide symporter, located in the outer cells of the thyroid will actively transport iodide ions inside. Stimulated by TSH, the thyroid now begins production of Triiodothyronine (T3) and Thyroxine (T4). To do this, the enzyme thyroperoxidase (TPO) oxidizes the iodide anion and incorporates it into thyroglobin which will later become T3 or T4. Most of I-131 can be eliminated in the urine. If the organism stops the intake of radioactive iodine, it can let the short half-life of the isotope decay it away. To prevent I-131 uptake entirely, it is possible to saturate the thyroid with stable I-127. This can be done with KI pills. “Radioactive I-131 from Fallout” http://www.cancer.gov/cancertopics/causes/i131 “EPA: Radionucleotides, Iodine” http://www.epa.gov/rpdweb00/radionuclides/iodine.html “Radiotoxicity of Iodine-123, Iodine-125, and Iodine- 131” http://jnm.snmjournals.org/cgi/reprint/33/12/2196.pdf “Thyroid Disorders” http://www.medicinenet.com/script/main/art.asp?articlekey=54416 “Seaweed as a model for iodide uptake and retention in the thyroid” http://www.endocrine-abstracts.org/ea/0007/ea0007p237.htm