Cycles in Nature PowerPoint
advertisement
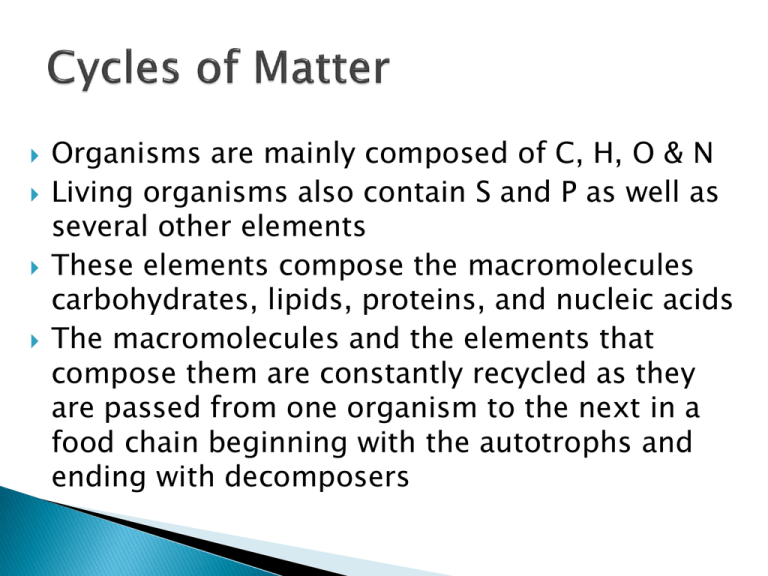
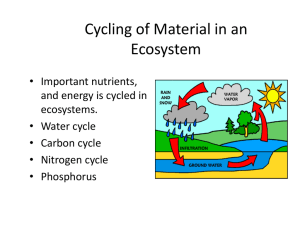
Organisms are mainly composed of C, H, O & N Living organisms also contain S and P as well as several other elements These elements compose the macromolecules carbohydrates, lipids, proteins, and nucleic acids The macromolecules and the elements that compose them are constantly recycled as they are passed from one organism to the next in a food chain beginning with the autotrophs and ending with decomposers Cycles of matter that involve biological, geological, and chemical processes Biological – involves eating, breathing, processing food, and eliminating waste Geological – volcanic eruptions, formation and breakdown of rock, and major movements within and below the surface of the earth Chemical – formation of clouds and precipitation, the flow of running water, and lightning Human activity – mining, burning fossil fuels and wood, clearing land for building or farming, and the manufacture and use of fertilizers affect the cycles of matter on a global scale Water continuously moves between the oceans, atmosphere, and the land – sometimes outside living organisms and sometimes inside them Transpiration Evaporation Condensation Precipitation Run-Off Water Vapor Macromolecules such as carbohydrates, lipids, proteins, and nucleic acids are NUTRIENTS that organisms need to survive There are specific element cycles for Carbon, Nitrogen, and Phosphorus Oxygen and Hydrogen do not have their own cycles as they are combined with other elements in the Water, Carbon, Nitrogen, and Phosphorus cycles Carbon is found in all four macromolecules: Carbohydrates, Lipids, Proteins, and Nucleic Acids Organic Compounds = Living Compounds ◦ Carbon-Based Life ◦ Fossil Fuels Photosynthesis and Cellular Respiration ◦ Water + Carbon Dioxide + Sunlight = Oxygen + Glucose ◦ Glucose + Oxygen = Water + Carbon Dioxide + ATP Nitrogen is found in the macromolecules Proteins and Nucleic Acids Nitrogen gas (N2) makes up 78% of the atmosphere ◦ This Nitrogen is not in a form usable by organisms ◦ Nitrogen Fixation – rhizobacteria and mycorrhizae Convert nitrogen gas into ammonia (NH3) Other bacteria convert the ammonia to nitrites (NO2-) and nitrates (NO3-) that are usable by autotrophs ◦ Denitrification – accomplished by bacteria Convert nitrates into nitrogen gas N2 NH3 or NH4+ (nitrogen fixing bacteria) Atmospheric nitrogen is converted to ammonia or ammonium ion by nitrogen-fixing bacteria that live in legume root nodules or in soil, or atmospheric nitrogen is converted to nitrogen oxides by lightening. NH3 or NH4+ (soil bacteria) NO2- NO2- + H2O Ammonia and Ammonium are oxidized by soil bacteria first to nitrite ions and then to nitrate ions NH3 or NO3- or NO2 NO3- + 2H+ (denitrifying bacteria) N2 After plants have taken up nitrogen from the soil in the form of nitrate ions, the nitrogen is passed along the food chain. When those plants and animals dies, bacteria and fungi take up and use some of the nitrogen from the plant/animal protein and other nitrogen containing molecules. The remaining nitrogen is released as ammonium ions or ammonia gas. Denitrifying bacteria convert some ammonia, nitrite, and nitrate back to nitrogen gas, which returns to the atmosphere. N2 + 3H2 2NH3 Heat + CH4 + H2O CO + H2O 3H2 + CO H2 + CO2 The phosphorus cycle does not have a significant atmospheric component Phosphorus remains mostly on land in the form of phosphate rock and soil minerals and in the ocean as dissolved phosphate and phosphate sediment Phosphorus is not as abundant as C, H, O, N, and S Phosphorus is found in the macromolecules Lipids, Proteins, and Nucleic Acids