Presentation - Hollings Cancer Center
advertisement

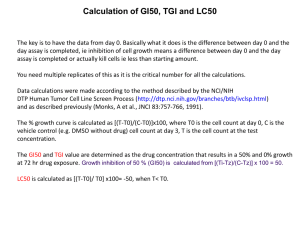
Cancer Centers Program Update and Future Directions Linda K. Weiss, PhD Director, Office of Cancer Centers Cancer Center Administrators Forum April 5, 2011 http://cancercenters.cancer.gov weissl@mail.nih.gov Overview • • • • • • • • Portfolio FY 2010 Funding, Parent Grant and Special Initiatives FY 2011 Budget Organizational Changes Database, Website, and Centers Report Miscellaneous Items Program Announcement 11-005 Cancer Center Support Grant Guidelines: Where Next? Portfolio • Geographic Distribution of Cancer Centers – 66 Centers in 33/50 states and DC – 40 Comprehensive: 23 states and DC – 26 Cancer Centers: 17 states: 17 states CCSG Funding By Application Type, FY2010, $271,490,840 Total Costs, Parent Grant New 1%, $1.5 M NonCompeting $177.0 M, 65% Competing $43.2 M, 16% Extensions $50.0 M, 18% FY 10 Funding for Special Initiatives • Supplemental Initiatives through OCC: $7.4 M – – – – – – Ca BIG: 0.8 M AMC: 1.0 M CFAR: 1.5 M NCCCP: 0.8 M RaPID: 3.0 M Miscellaneous: 0.6 M • Additional Supplements – CTRP (NCI CCCT): 5.2 M – Core Consolidation (NCRR): 8.0 M FY 2011 Budget • Centers Baseline Budget – Continuing Resolution now in effect until 4/8 – Centers budget has not been finalized – T3/5 funded under NIH policy at 90% – T1/2 budget pending • Anticipated Supplements – Clinical Investigator Team Leadership Award – Clinical Trials Reporting Program – CFAR – CC Pilot Project Awards – AMC – CC Pilot Project Awards Organizational Changes • New NCI Leadership – – – – – Dr. Harold Varmus, Director Dr. Douglas Lowy, Deputy Director Dr. Peter Greenwald, Associate Director for Prevention and Control John Czajkowski, Deputy Director for Management Searches underway • • • • Associate Director for Clinical Research Director Center for Center for Cancer Genomics Director for Center for Global Health Director for Division of Cancer Prevention • Office of Cancer Centers New Staff – Nga Nguyen, Program Analyst – Searches underway • Program Director • IT Database Specialist Database, Website and Centers Report • Summaries – Send as before to ccsgdata@mail.nih.gov – Send content questions to your program director • Website Updates – Send center name, address, directorship changes to your program director – More complex changes will be deferred • Centers Report – Nearing completion – Some information will be web-based (e.g., publications, clinical trials) Miscellaneous Items • Include PMCID numbers with publications list • Large carryover balances are discouraged, particularly as a repeated pattern; there is no guaranteed approval • Fewer administrative supplements (extensions with funds) than in previous years, no ‘recycling’ • New programs and shared resources can’t be established with CCSG funds during non-competing years; realigned programs must be approved by staff Program Announcement 11-005 • Overview Opportunity Announcement – Effective September 25, 2010, NIH required that all mechanisms have a Funding Opportunity Announcement in the NIH Guide for Grants and Contracts – All applications will have to respond to the PAR – The PAR, new Guidelines, and a comparison of 2008 and 2010 Guidelines are on our website. Program Announcement 11-005 • Face page of the application must indicate the PAR # • Resubmission applications (A1) now allowed within 37 months • New page limits for components are in effect • Appendices must be included in the application and can’t exceed 50 pages • Late materials must follow NIH policy – much more stringent limitations • Submission procedures for letter of approval and request for preliminary information have changed Program Announcement 11-005 • Applicants will no longer receive and respond to an administrative review letter • Applicants will no longer submit posters and updated summaries prior to the site visit; information not provided in the original application can be provided at the site visit. • The SRO can no longer selectively accept parts of the application • The application can be returned without review if it doesn’t meet guidelines requirements Program Announcement 11-005 – The 5 review criteria mandated by NIH (significance, investigator, innovation, approach and environment) are now incorporated into the Guidelines. – The 5 criteria will be incorporated into the evaluation of overall center impact but not individual components – Prior to the site visit assigned reviewers will submit criterion scores for the overall application on the 5 NIH mandated criteria. These scores will be in the summary statement, but won’t be discussed at the review. – The 6th year of funding for centers scoring in the outstanding range has been eliminated. – NIH policy and document citations have been updated. The CCSG Guidelines: Where Next? • Major Objectives – – – – Foster collaboration and integration Facilitate clinical and translational research Reduce the burden of the application process Provide new guidance on eligibility and budget requests The CCSG Guidelines: Where Next? • Foster Collaboration and Integration – Recognize research collaborations that extend beyond the walls of the center, including ‘hand-offs’ to other mechanisms or entities that move scientific findings forward – Encourage productive interactions with other NCI and NIH programs – Allow sharing of core services across centers – Eliminate the benchmark ratio The CCSG Guidelines: Where Next? • Facilitate Clinical and Translational Research – Make Clinical Trial and Data Management (the CTO) a separate component and broaden options for support – Harmonize guidelines to recognize leadership and participation in the cooperative groups – Recognize variety and quality in clinical and translational research – Promote team contributions in clinical research The CCSG Guidelines: Where Next? • Reduce the Burden of the Application Process – Eliminate • multiple redundancies across components • detailed capacity and usage tables in shared resource components • data on non-aligned members • requirements for program meeting agendas – Streamline • clinical and other data requirements • requirements for the administrative component The CCSG Guidelines: Where Next? • Provide New Guidance on Eligibility and Budget – Raise eligibility minimum, e.g., from $4 to $10 M – Budget Requests • Base request on percent over prior award or a minimum level or ‘floor’ whichever is greater, e.g., – 20% over last T5 award or $2 M DC • Allow for exceptions, e.g., – First T2 – First application after no-cost extension – Major expansion of research • Funding level would still be dependent on merit and NCI budget The CCSG Guidelines: Where Next? • Other Potential Changes – More specificity in language for consortium centers – Expansion/redefinition of staff investigator category – Modification in criteria for Protocol specific resaerch support – Elimination of the limited site visit The CCSG Guidelines: Where Next? • Process – Collaborators/Consultants • NCI – Review – Grants Administration – Senior leadership • Cancer Centers – Approvals • NCI senior leadership • NCAB Subcommittee on Cancer Centers • NIH (to include a new PAR) • Tentative timeline for implementation: 2012 QUESTIONS?Questions?