2012_ISSN_poster
advertisement
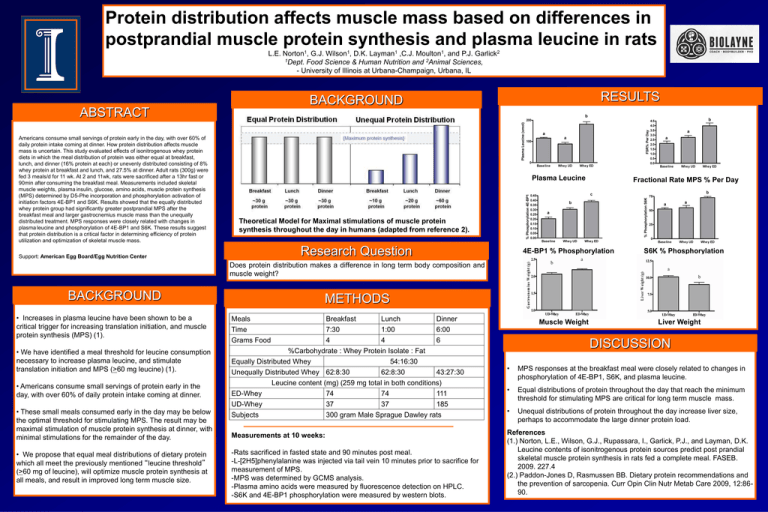
Protein distribution affects muscle mass based on differences in postprandial muscle protein synthesis and plasma leucine in rats L.E. Norton1, G.J. Wilson1, D.K. Layman1 ,C.J. Moulton1, and P.J. Garlick2 1Dept. Food Science & Human Nutrition and 2Animal Sciences, - University of Illinois at Urbana-Champaign, Urbana, IL RESULTS BACKGROUND ABSTRACT Americans consume small servings of protein early in the day, with over 60% of daily protein intake coming at dinner. How protein distribution affects muscle mass is uncertain. This study evaluated effects of isonitrogenous whey protein diets in which the meal distribution of protein was either equal at breakfast, lunch, and dinner (16% protein at each) or unevenly distributed consisting of 8% whey protein at breakfast and lunch, and 27.5% at dinner. Adult rats (300g) were fed 3 meals/d for 11 wk. At 2 and 11wk, rats were sacrificed after a 13hr fast or 90min after consuming the breakfast meal. Measurements included skeletal muscle weights, plasma insulin, glucose, amino acids, muscle protein synthesis (MPS) determined by D5-Phe incorporation and phosphorylation activation of initiation factors 4E-BP1 and S6K. Results showed that the equally distributed whey protein group had significantly greater postprandial MPS after the breakfast meal and larger gastrocnemius muscle mass than the unequally distributed treatment. MPS responses were closely related with changes in plasma leucine and phosphorylation of 4E-BP1 and S6K. These results suggest that protein distribution is a critical factor in determining efficiency of protein utilization and optimization of skeletal muscle mass. Plasma Leucine Fractional Rate MPS % Per Day Theoretical Model for Maximal stimulations of muscle protein synthesis throughout the day in humans (adapted from reference 2). Research Question Support: American Egg Board/Egg Nutrition Center 4E-BP1 % Phosphorylation S6K % Phosphorylation Does protein distribution makes a difference in long term body composition and muscle weight? BACKGROUND • Increases in plasma leucine have been shown to be a critical trigger for increasing translation initiation, and muscle protein synthesis (MPS) (1). • We have identified a meal threshold for leucine consumption necessary to increase plasma leucine, and stimulate translation initiation and MPS (>60 mg leucine) (1). • Americans consume small servings of protein early in the day, with over 60% of daily protein intake coming at dinner. • These small meals consumed early in the day may be below the optimal threshold for stimulating MPS. The result may be maximal stimulation of muscle protein synthesis at dinner, with minimal stimulations for the remainder of the day. • We propose that equal meal distributions of dietary protein which all meet the previously mentioned “leucine threshold” (>60 mg of leucine), will optimize muscle protein synthesis at all meals, and result in improved long term muscle size. METHODS Meals Time Grams Food Breakfast Lunch Dinner 7:30 1:00 6:00 4 4 6 %Carbohydrate : Whey Protein Isolate : Fat Equally Distributed Whey 54:16:30 Unequally Distributed Whey 62:8:30 62:8:30 43:27:30 Leucine content (mg) (259 mg total in both conditions) ED-Whey UD-Whey Subjects 74 74 111 37 37 185 300 gram Male Sprague Dawley rats Measurements at 10 weeks: -Rats sacrificed in fasted state and 90 minutes post meal. -L-[2H5]phenylalanine was injected via tail vein 10 minutes prior to sacrifice for measurement of MPS. -MPS was determined by GCMS analysis. -Plasma amino acids were measured by fluorescence detection on HPLC. -S6K and 4E-BP1 phosphorylation were measured by western blots. Muscle Weight Liver Weight DISCUSSION • MPS responses at the breakfast meal were closely related to changes in phosphorylation of 4E-BP1, S6K, and plasma leucine. • Equal distributions of protein throughout the day that reach the minimum threshold for stimulating MPS are critical for long term muscle mass. • Unequal distributions of protein throughout the day increase liver size, perhaps to accommodate the large dinner protein load. References (1.) Norton, L.E., Wilson, G.J., Rupassara, I., Garlick, P.J., and Layman, D.K. Leucine contents of isonitrogenous protein sources predict post prandial skeletal muscle protein synthesis in rats fed a complete meal. FASEB. 2009. 227.4 (2.) Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009, 12:8690.