ppt - Georgetown University
advertisement
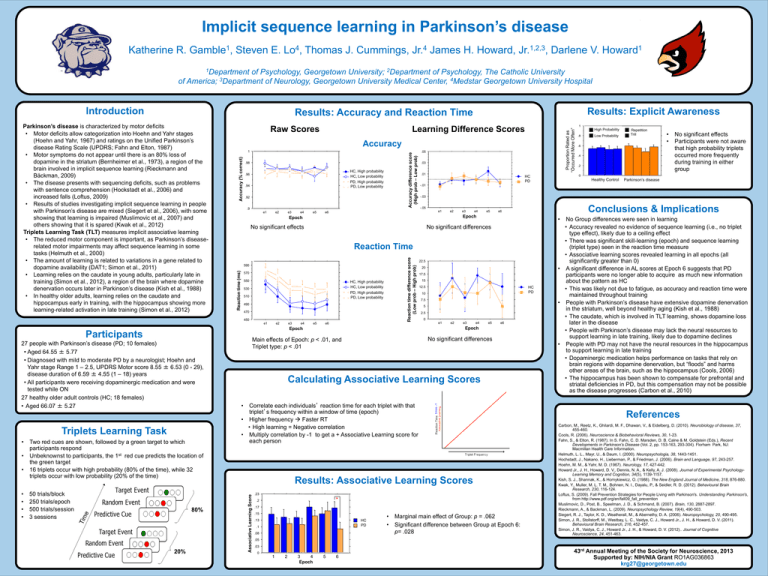
Implicit sequence learning in Parkinson’s disease Katherine R. 1 Gamble , Steven E. 4 Lo , Thomas J. Cummings, 4 Jr. James H. Howard, 1,2,3 Jr. , Darlene V. 1 Howard 1Department of Psychology, Georgetown University; 2Department of Psychology, The Catholic University of America; 3Department of Neurology, Georgetown University Medical Center, 4Medstar Georgetown University Hospital 1 Results: Accuracy and Reaction Time .8 Raw Scores Accuracy difference score (High prob – Low prob) Accuracy (% correct) .98 HC, High probability Control, High HC, LowLow probability Control, PD, High PD, High probability PD, Low probability PD, Low .96 .94 .92 .9 e2 e3 e4 e5 • • • • • 50 trials/block 250 trials/epoch 500 trials/session 3 sessions 80% 20% .2 0 .6 .6.6 .6 0 Control .01 Control HC Reaction time difference score (Low prob – High prob) 550 HC, High probability Control, High HC, LowLow probability Control, PD, High PD, High probability PD, Low probability PD, Low 530 510 490 470 e2 e3 e4 e5 e6 Repetition Trill PD 0 • • No significant effects Participants were not aware High High High High Low Low Low Low that high probability triplets Repetition Repetition Repetition Repetition occurred more frequently TrillTrill Trill Trill during training in either group 00 0 Control Control Control Control Healthy Control PD PD PD PD Parkinson’s disease -.03 Conclusions & Implications -.05 e2 e3 e4 e5 e6 • 22.5 20 17.5 15 12.5 Control HC PD PD 10 7.5 5 2.5 0 e1 e2 e3 e4 e5 e6 Epoch No significant differences Main effects of Epoch: p < .01, and Triplet type: p < .01 LowProbability Low Repetition Repetition Trill Trill -.01 No significant differences 570 PD PD PD Epoch 590 High HighProbability .4 .4.4 .4 Reaction Time Calculating Associative Learning Scores No Group differences were seen in learning • Accuracy revealed no evidence of sequence learning (i.e., no triplet type effect), likely due to a ceiling effect • There was significant skill-learning (epoch) and sequence learning (triplet type) seen in the reaction time measure • Associative learning scores revealed learning in all epochs (all significantly greater than 0) • A significant difference in AL scores at Epoch 6 suggests that PD participants were no longer able to acquire as much new information about the pattern as HC • This was likely not due to fatigue, as accuracy and reaction time were maintained throughout training • People with Parkinson’s disease have extensive dopamine denervation in the striatum, well beyond healthy aging (Kish et al., 1988) • The caudate, which is involved in TLT learning, shows dopamine loss later in the disease • People with Parkinson’s disease may lack the neural resources to support learning in late training, likely due to dopamine declines • People with PD may not have the neural resources in the hippocampus to support learning in late training • Dopaminergic medication helps performance on tasks that rely on brain regions with dopamine denervation, but “floods” and harms other areas of the brain, such as the hippocampus (Cools, 2006) • The hippocampus has been shown to compensate for prefrontal and striatal deficiencies in PD, but this compensation may not be possible as the disease progresses (Carbon et al., 2010) • Correlate each individuals’ reaction time for each triplet with that triplet’s frequency within a window of time (epoch) • Higher frequency Faster RT • High learning = Negative correlation • Multiply correlation by -1 to get a + Associative Learning score for each person Results: Associative Learning Scores Associative Learning Score • .2 .8 .8.8 .8 Control e1 Epoch Two red cues are shown, followed by a green target to which participants respond Unbeknownst to participants, the 1st red cue predicts the location of the green target 16 triplets occur with high probability (80% of the time), while 32 triplets occur with low probability (20% of the time) .4 Low 11 1 .2 .2.2 .2 No significant effects Participants • .4 .03 Epoch e1 Triplets Learning Task 1 .05 e6 450 27 people with Parkinson’s disease (PD; 10 females) • Aged 64.55 ± 5.77 • Diagnosed with mild to moderate PD by a neurologist; Hoehn and Yahr stage Range 1 – 2.5, UPDRS Motor score 8.55 ± 6.53 (0 - 29), disease duration of 6.59 ± 4.55 (1 – 18) years • All participants were receiving dopaminergic medication and were tested while ON 27 healthy older adult controls (HC; 18 females) • Aged 66.07 ± 5.27 .6 .6 1 e1 High Learning Difference Scores Accuracy Reaction time (ms) Parkinson’s disease is characterized by motor deficits • Motor deficits allow categorization into Hoehn and Yahr stages (Hoehn and Yahr, 1967) and ratings on the Unified Parkinson’s disease Rating Scale (UPDRS; Fahn and Elton, 1987) • Motor symptoms do not appear until there is an 80% loss of dopamine in the striatum (Bernheimer et al., 1973), a region of the brain involved in implicit sequence learning (Rieckmann and Bäckman, 2009) • The disease presents with sequencing deficits, such as problems with sentence comprehension (Hockstadt et al., 2006) and increased falls (Loftus, 2009) • Results of studies investigating implicit sequence learning in people with Parkinson’s disease are mixed (Siegert et al., 2006), with some showing that learning is impaired (Muslimovic et al., 2007) and others showing that it is spared (Kwak et al., 2012) Triplets Learning Task (TLT) measures implicit associative learning • The reduced motor component is important, as Parkinson’s diseaserelated motor impairments may affect sequence learning in some tasks (Helmuth et al., 2000) • The amount of learning is related to variations in a gene related to dopamine availability (DAT1; Simon et al., 2011) • Learning relies on the caudate in young adults, particularly late in training (Simon et al., 2012), a region of the brain where dopamine denervation occurs later in Parkinson’s disease (Kish et al., 1988) • In healthy older adults, learning relies on the caudate and hippocampus early in training, with the hippocampus showing more learning-related activation in late training (Simon et al., 2012) Results: Explicit Awareness .8 Proportion Rated as “Occurred More Often” Introduction 1 .23 * .2 .17 .15 .13 Control HC PD PD .1 .08 • • Marginal main effect of Group: p = .062 Significant difference between Group at Epoch 6: p= .028 References Carbon, M., Reetz, K., Ghilardi, M. F., Dhawan, V., & Eidelberg, D. (2010). Neurobiology of disease, 37, 455-460. Cools, R. (2006). Neuroscience & Biobehavioral Reviews, 30, 1-23. Fahn, S., & Elton, R. (1987). In S. Fahn, C. D. Marsden, D. B. Calne & M. Goldstein (Eds.), Recent Developments in Parkinson's Disease (Vol. 2, pp. 153-163, 293-304). Florham Park, NJ: Macmillan Health Care Information. Helmuth, L. L., Mayr, U., & Daum, I. (2000). Neuropsychologia, 38, 1443-1451. Hochstadt, J., Nakano, H., Lieberman, P., & Friedman, J. (2006). Brain and Language, 97, 243-257. Hoehn, M. M., & Yahr, M. D. (1967). Neurology, 17, 427-442. Howard Jr., J. H., Howard, D. V., Dennis, N. A., & Kelly, A. J. (2008). Journal of Experimental PsychologyLearning Memory and Cognition, 34(5), 1139-1157. Kish, S. J., Shannak, K., & Hornykiewicz, O. (1988). The New England Journal of Medicine, 318, 876-880. Kwak, Y., Muller, M. L. T. M., Bohnen, N. I., Dayalu, P., & Seidler, R. D. (2012). Behavioural Brain Research, 230, 116-124. Loftus, S. (2009). Fall Prevention Strategies for People Living with Parkinson's. Understanding Parkinson's, from http://www.pdf.org/en/fall09_fall_prevention Muslimovic, D., Post, B., Speelman, J. D., & Schmand, B. (2007). Brain, 130, 2887-2897. Rieckmann, A., & Backman, L. (2009). Neuropsychology Review, 19(4), 490-503. Siegert, R. J., Taylor, K. D., Weatherall, M., & Abernethy, D. A. (2006). Neuropsychology, 20, 490-495. Simon, J. R., Stollstorff, M., Westbay, L. C., Vaidya, C. J., Howard Jr., J. H., & Howard, D. V. (2011). Behavioural Brain Research, 216, 452-457. Simon, J. R., Vaidya, C. J., Howard Jr., J. H., & Howard, D. V. (2012).. Journal of Cognitive Neuroscience, 24, 451-463. .05 .03 0 e1 1 e2 2 e3 3 e4 4 Epoch e5 5 e6 6 43rd Annual Meeting of the Society for Neuroscience, 2013 Supported by: NIH/NIA Grant RO1AG036863 krg27@georgetown.edu











