NIH
advertisement

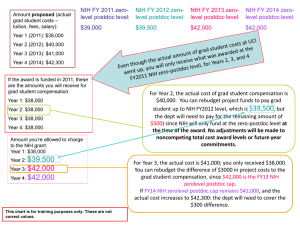
October 2011 Sally J. Rockey, PhD Deputy Director for Extramural Research 1 Institutes of Health National “To win the future, America needs to out-educate, out-innovate, and out-build the rest of the world.” President Barack Obama, Weekly Address February 5, 2011 NIH: Steward of Biomedical & Behavioral Research for the Nation “Science in pursuit of fundamental knowledge about the nature and behavior of living systems . . . and the application of that knowledge to extend healthy life and reduce the burdens of illness and disability 3 NIH Appropriation History vs. Actual Purchasing Power - BRDPI* 4 *Adjustment to FY 1998 dollars based on Biomedical Research & Development Price Index (BRDPI) NIH Extramural & Intramural Funding FY 2011 Enacted: $30.9 Billion Spending at NIH $5.0 B – $3.3 B Intramural Research – $1.5 B Research Management & Support – $0.2 B Buildings and Facilities, Other 16% Spending Outside NIH $25.9 B 84% – Supports over 325,000 Scientists & Research Personnel – Supports over 3,000 Institutions FY 2010 Percent Distribution of Basic and Clinical Research R&D Facilities 0.3% Training & Overhead 2.6% Applied Research (Other) 10.5% Applied Research (Clinical) 34.6% Basic Research 52.0% Research Project Grants Applications, Awards, and Success Rates 7 "Cutting the deficit by gutting our investments in innovation and education is like lightening an overloaded airplane by removing its engine. It may make you feel like you're flying high at first, but it won't take long before you'll feel the impact.“ President Obama 8 Bridging the Gap Directions for NIH Supported Research Basic Research 9 Drugs The Therapeutic Development Pipeline 10 HTS Probe to Lead NIH Molecular Libraries Initiative PreClinical NIH RAID NIH Supported Basic Research Assay Dev. NIH TRND Disease Target ID FDA IND FDA Ph. 0 Ph. I Ph. II Ph. III Review Pharma, Biotech, NIH Clinical Center, CTSAs New NIH FDA Partnerships Creation of the National Center for Advancing Translational Sciences (NCATS) To catalyze the development of innovative methods and technologies that will enhance the development, testing, and implementation of diagnostics and therapeutics across a wide range of human diseases and conditions. Updated 5.18.11 NCATS: Challenges & Opportunities • Deluge of new discoveries of potential targets • Unmet therapeutic needs for many conditions, especially rare and neglected diseases • Need to view drug development pipeline as a scientific problem – ripe for experimentation and process engineering NCATS: Functions Improve the processes of diagnostics and therapeutics development, testing, and implementation by: • Experimenting with innovative approaches in an open-access model • Choosing therapeutic projects to evaluate these innovative approaches • Promoting interactions to advance the field of regulatory science Catalyze the development and implementation of new diagnostics and therapeutics by: • Encouraging collaborations across all sectors • Providing resources to enable diagnostics and therapeutic development and implementation • Enhancing training in relevant disciplines NCATS will: • Facilitate – not duplicate – other translational research activities supported by NIH • Complement – not compete with – the private sector • Reinforce – not reduce – NIH’s commitment to basic research Better Ways to Predict Drug Safety New NIH-DARPA-FDA Collaboration • Part of President’s “Lab to Market” initiatives • Goal: Develop chip to screen for safe, effective drugs ▫ Liver, heart, lung, other cell types ▫ Designed for multiple different readouts • NIH, DARPA to commit ~$70 million each over 5 years • FDA to offer guidance • Fall: First Requests for Proposals ▫ Seeking best ideas in engineering, biology, toxicology Future of the Biomedical Research Workforce 16 NIH Establishes Working Group to Examine the Future Biomedical Workforce Using appropriate expertise from NIH and external sources, the group will develop a model for a sustainable and diverse U.S. biomedical research workforce that can inform decisions about training of the optimal number of people for the appropriate types of positions that will advance science and promote health. Based on this analysis and the input gathered from the extramural community, the committee will make recommendations for actions that NIH should take to support a future sustainable biomedical infrastructure. 17 Distribution of FY2009 RPG Support to Principal Investigators 18 Number of RPG Awards per PI – Top 20% 19 Distribution of FY2009 RPG Funding Across Institutions 100% Percent of All RPG Funding 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 0% 10% 20% 30% 0 120 240 360 40% 50% 60% 480 600 720 Percent / Number of Organizations 70% 80% 90% 100% 840 960 1080 1200 Distribution of Principal Investigators by Degree Type 21 36.0 34.0 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 Age at First R01 Award Age at First R01 Equivalent Award from NIH FY 1980 to 2010 48.0 46.0 44.0 42.0 40.0 38.0 MD-PhD MD Only PhD Only 32.0 Fiscal Year 20 NIH Exceeds New Investigator Goals since FY 2007 Used the rolling average of the previous 5 years Used equalized success rates between new and established R01-Equivalent awards include R01, R23, R29, and R37 grants. 17 R01-Equivalent Grants Funding Rates by Gender and Type of Application 24 Race and Ethnicity of Reporting Principal Investigators on Research Project Grants 2009 25 26 Career Stages of Funding Programs Kirschstein-NRSA Training Grants and Fellowships - Numbers 27 Estimated Number of NIH-Supported Graduate Students Sources: NSF Survey of Graduate Students and Postdoctorates in Science and Engineering and the NIH Data Book 28 Estimated Number of NIH-Supported Postdocs Sources: NSF Survey of Graduate Students and Postdoctorates in Science and Engineering and the NIH Data Book 29 Potential Levers for Dealing with Budget Challenges 30 Trim Spending Across the Board • No systematic changes • Annual belt-tightening for all programs • Consequences: ▫ Darwinian approach; survival of the fittest ▫ Success rates will likely continue to fall ▫ Risk of reduced emphasis on innovation as applicants play it safe to get through peer review Evaluate, Rearrange Research Portfolio • Focus on scientific priorities • Conduct rigorous evaluation of entire NIH research portfolio • Reset priorities in focused, intentional way to: ▫ eliminate duplications ▫ reduce support for less innovative research ▫ increase support for highly innovative research Change Ways of Managing NIH Resources • Investigators: ▫ Limit PI’s # of Research Program Grant (RPG) awards ▫ Limit PI’s total amount of awards ▫ Limit size of awards ▫ Limit PI salaries • NIH Intramural • Grantee Institutions ▫ Limit cost-recovery (indirect costs) ▫ Reduce burdens on institutions Other Issues of Interest – Common Rule Federal Policy for the Protection of Human Subjects (in place since 1991) HHS and OSTP issued an Advanced Notice of Proposed Rulemaking on July 26, 2011 requesting comment on proposed changes (http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/pdf/2011-18792.pdf) Changes proposed in the following areas: 1. Revising the existing risk-based framework to more accurately calibrate the level of review to the level of risk. 2. Using a single Institutional Review Board review for all domestic sites of multi-site studies. 3. Updating the forms and processes used for informed consent. 4. Establishing mandatory data security and information protection standards for all studies involving identifiable or potentially identifiable data. 5. Implementing a systematic approach to the collection and analysis of data on unanticipated problems and adverse events across all trials to harmonize the complicated array of definitions and reporting requirements, and to make the collection of data more efficient. 6. Extending federal regulatory protections to apply to all research conducted at U.S. institutions receiving funding from the Common Rule agencies. 7. Providing uniform guidance on federal regulations • Comments will be received through October 26, 2011 (see http://www.hhs.gov/ohrp/humansubjects/submitanprmcomment.html) 34 Find Grants Info at: http://www.grants.nih.gov/ Finding Funded Research: http://RePORT.NIH.Gov RePORT & RePORTER (Formally known as CRISP) •Quick access to “Frequently Requested Reports” (e.g. Funding by State, Funding by Award Mechanism, etc.) •Efficient search tools for locating data and reports •Links to funding estimates for certain research areas, conditions, & diseases. •Includes ARRA-specific data queries 36 37