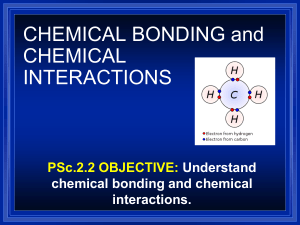
Valence electrons form chemical bonds
advertisement

Valence Electrons: TEKS 8.5B To the teacher: • This CPO Science PowerPoint presentation is designed to guide you through the process of presenting the lesson to your students. The presentation uses a 5-E teaching model: Engage, Explore, Explain, Elaborate, and Evaluate. • The PowerPoint Slide notes indicate where you may want to bring in various lesson elements such as quizzes, readings, investigations, animations, and practice materials. Additional science background information is provided in the slide notes where appropriate. You can view these notes by selecting “View,” then “Normal.” You will see the notes pane at the bottom of the PowerPoint workspace. Additionally, the slide notes are available as a separate document, accessible from the lesson home page. • The slides that follow are intended for classroom use. Valence Electrons: TEKS 8.5B What do aspirin, vinegar, and plastic wrap have in common? • Aspirin, vinegar, and plastic wrap are all compounds made from different combinations of the same three atoms: carbon, hydrogen, and oxygen. By themselves, these atoms cannot reduce pain, season food, or keep candy clean. But when these atoms chemically combine in certain ways to form compounds, they can be used in may ways. • In this module, you will learn how and why atoms combine to form an amazing variety of materials. Valence Electrons: TEKS 8.5B The periodic table Valence Electrons: TEKS 8.5B Time to investigate! • Complete the lesson investigation: – Chemical Bonds Valence Electrons: TEKS 8.5B Identifying elements • The number of protons in an element determines that element’s identity. • The number of protons is called the element’s atomic number. The atomic number is listed under the element’s symbol on the periodic table. • All helium atoms have two protons, and all boron atoms have five. • How many protons does a fluorine atom have? Valence Electrons: TEKS 8.5B Electron arrangements • A neutral atom has the same number of electrons and protons. • The electrons fill the innermost energy level first. It can hold two electrons. The second and third energy levels hold eight electrons each. • Look at the electron arrangement for boron. Notice that the electrons in the second energy level spread out, since they repel each other. • Can you draw the electron arrangement for fluorine? ? Valence Electrons: TEKS 8.5B Valence electrons form chemical bonds • The electrons in the highest unfilled energy level are called valence electrons. • Valence electrons participate in chemical bonding. • Elements heavier than boron form bonds to get eight electrons in their outer shell. • Elements with five protons or less need to get two electrons in their outer shell. Hydrogen is a special case, as it can have zero or two electrons in its outer shell. Valence Electrons: TEKS 8.5B How reactive is an element? • In science, reactive means that an element readily forms chemical bonds. • The closer an element is to having the same number of valence electrons as a noble gas, the more reactive the element is. • Noble gases are not usually reactive because their valence shells are already filled. Valence Electrons: TEKS 8.5B Time for Practice! • Complete the lesson practice activity: – Dot Diagrams Valence Electrons: TEKS 8.5B Show what you know! • Try the lesson’s interactive quiz, or complete a quiz that your teacher can print out for you. • Hint: – You might want to review your lesson reading piece one more time before trying the quiz.



![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)