Chapter 11 section 2
advertisement

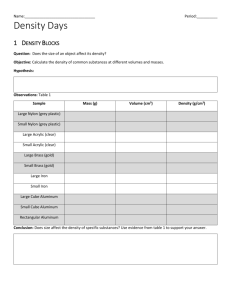
Chapter 11 section 2 Floating and Sinking Density • Density=mass volume Calculating Density 1. A sample of liquid has a mass of 24g and a volume of 16mL. What is its density? 2. A piece of metal has a mass of 43.5g and a volume of 15cm3. What is its density? • Comparing Densities of substances, to compare the densities of different substances, you can place them in the same container, the densest will sink at the bottom and the least dense will be on the top • Changing densities Since Density=mass/volume changing the mass and/or the volume will change the density of the substance Buoyancy • Buoyancy is the ability of objects to float. • The buoyant force is an upward force that fluids exert on all matter. Buoyant force acts in the direction opposite to the force of gravity, so it makes an object feel lighter. • Fluid exert pressure on all directions of submerged object. • If the weight of submerged object is greater than buoyant force the object will sink, If the weight of submerged object is equal to buoyant force, the object will not sink. (Remember we also use density to explain why objects sink or float). Archimedes’ Principle • Archimedes’ Principle states that the buoyant force acting on a submerged object is equal to the weight of the volume of fluid displaced by the object. • Submarines sink when its weight is greater than the buoyant force. It rises when its weight is less than the buoyant force. • Ships; a solid block of steel sinks in water. A steel ship with the same weight floats on the surface since the ship displaces more water than block of steel, a greater buoyant force acts on the ship.